Article Text
Abstract
Background Infliximab (IFX) is a monoclonal antibody against tumour necrosis factor α that is effective for treating spondyloarthritis (SpA). However, after initial success of the drug some patients lose responsiveness or develop infusion reactions, which may be related to the development of antibodies against the drug.
Objective To investigate the clinical relevance of antibodies to infliximab (ATI) formation in patients with SpA undergoing IFX treatment over a prolonged period.
Methods 94 patients with SpA treated with IFX from 1999 to 2010 were studied. Their clinical characteristics, serum trough IFX levels and ATI status were evaluated for a mean of 6.99 (95% CI:6.28 to 7.7) years. Clinical activity and improvement were measured using the Ankylosing Spondylitis Disease Activity Score (ASDAS): inactive <1.3, moderate ≥1.3 and <2.1, high ≥2.1–≤3.5, and very high >3.5 at three time points (6 months, 12 months and >4 years).
Results ATI were detected in 24 (25.5%) patients. The patients with ATI had higher ASDAS scores than those without ATI (2.55±0.89 vs 1.79±1.04, p=0.038 at 6 months; 1.95±0.67 vs 1.67±0.71, p=0.042 at 1 year; 2.52±0.99 vs 1.53±0.81, p=0.024 at >4 years). Eleven patients (12%) developed infusion-related reactions, and of these, ATI were present in eight patients (73%). The patients with infusion-related reactions had higher ATI titres (median 12 931 AU/ml, IQR 853–82 437) vs median 2454 AU/ml, IQR 449–7718, p=0.028) and shorter survival (4.25 years vs 8.19 years, p<0.001). ATI development occurred more frequently in the patients not receiving methotrexate (20/58 (34.5%) vs 4/36 (11.1%), p=0.011).
Conclusion In patients with SpA treated with IFX, ATI formation is associated with a poor clinical response, the appearance of infusion reactions and the discontinuation of treatment.
Statistics from Altmetric.com
Introduction
Spondyloarthritis (SpA) is a heterogeneous group of diseases consisting of ankylosing spondylitis (AS), psoriatic arthritis (PsA), arthritis related to inflammatory bowel disease (IBD), reactive arthritis, a subgroup of juvenile idiopathic arthritis and undifferentiated spondyloarthritis.1 Until recently, the main treatment for SpA was based on non-steroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs (DMARDs) and physical therapy. Although DMARDs such as methotrexate (MTX) and sulfasalazine can be useful for treating the peripheral joint manifestations of SpA, they have no demonstrated efficacy for treating the axial manifestations2,–,5; however, several studies have demonstrated the efficacy of biological drugs such as antitumour necrosis factor α (anti-TNFα) for treating patients with SpA.6,–,13
All biological drugs can induce an immune response, with the structure of the anti-TNF agent being a decisive factor in its immunogenicity. Infliximab (IFX) is a chimeric monoclonal IgG1 antibody against TNF that has been approved for treating moderate to severe SpA. The drug elicits a response that is inferior to that obtained with conventional treatment. Although the efficacy of IFX against SpA has been shown in large, randomised clinical trials,14,–,17 it is known that >30% of patients with AS fail to respond or lose their responsiveness.18 Part of this treatment failure can be explained by the development of antidrug antibodies.18,–,20
Until now, few studies have been published on treating SpA with IFX. Two of these studies have been conducted in patients with AS and have investigated the clinical response in relation to antibodies to IFX (ATI) formation after ≥1 year.18 ,20 In this study, we analysed the clinical consequences of ATI formation in a group of patients with SpA (some of whom had conditions other than AS) who were treated with IFX over long periods. At the same time, there is increasing interest in examining the influence of MTX on patients with SpA treated with IFX.21 ,22 In our group of patients with SpA, we assessed whether concomitant immunosuppressive treatment with MTX plays a role in ATI production.
Patients and methods
Patients and serum samples
A total of 94 patients with SpA (50 AS, 12 undifferentiated spondyloarthritis, 22 PsA and 10 SpA associated with IBD) who had not received previous biological treatment were included. The patients were enrolled at the department of rheumatology of La Paz University Hospital. This was an ambispective observational study that was approved by the La Paz Hospital ethics committee. The patients signed an informed consent form. Serum samples (a total of 2116) were collected at the time of infusion. The retrospective study period covered the years from 1999 to 2008, and the prospective study period extended from 2009 to 2010. All of the patients with AS fulfilled the revised New York criteria. The patients with PsA fulfilled the GRAPPA (Group of Research and Assessment of Psoriasis and Psoriatic Arthritis) group criteria. All the patients with IBD fulfilled the ESSG (European Spondylarthropathy Study Group) criteria. At the time of inclusion, all patients had evidence of active disease, as indicated by their mean Ankylosing Spondylitis Disease Activity Score (ASDAS) of 3.08±1.31 (mean ± SD). All the patients were given intravenous infusions of 5 mg/kg IFX at 0, 2 and 6 weeks and then every 8 weeks thereafter. Every 6 months, disease activity was measured using the ASDAS (in the retrospective group of patients, the clinical response data were extrapolated from the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)).23 Six months, 1 year and >4 years (mean 5.9 years, SD 2 years) were chosen as the time points that would be used to represent the clinical course. Infusion reactions were defined as events appearing during infusion that required either cessation of the drug infusion or the administration of parenteral medication.
Blood samples were collected at baseline and immediately before the 2- and 6-week infusions and every 8 weeks thereafter. Precise timing was required to compare the results because serum IFX may become undetectable over longer time intervals owing to normal drug pharmacokinetics rather than the formation of ICs with ATI. Therefore, samples taken more than 9 weeks after the previous infusions were not used in the study. The serum samples were stored at −80°C until measurement of the IFX and ATI. Serum samples from all patients at each of the three studied time points (6 months, 1 year and >4 years) could not be obtained. We obtained samples from 56 patients immediately after they had begun treatment, from nine patients in the first year but after starting IFX treatment and from 29 patients after the first year of anti-TNF treatment. A minimum of five samples and a maximum of 32 samples per patient were collected. In all patients from whom baseline samples were available, the IFX and ATI concentrations were <10 ng/ml and 50 arbitrary units per millilitre (AU/ml), respectively.
Serum IFX assay
The serum IFX levels were determined by a sandwich ELISA, as has been described previously.24 ,25 The cut-off values were established from the serum samples of 150 healthy blood donors and 100 patients with rheumatoid arthritis (RA) who had never received IFX (70% of whom were rheumatoid factor positive). Serum IFX levels >10 ng/ml (the mean + 6 SD of the control group) were considered positive.
Antibodies to infliximab (ATI) assay
ATI were detected using a two-site (bridging) ELISA as has been previously described.25 The cut-off point for the presence of ATI in patient serum samples was established at 50 AU/ml (the mean + 6 SD of the same control group used for the IFX measurements).
Statistical analysis
The descriptive statistics consisted of the mean, SD, median (Mdn) and IQR. The statistical analyses were performed using the Statistical Package for the Social Sciences, version 10.0 (SPSS, Chicago, Illinois, USA). The frequency data were compared using the Pearson χ2 and Fisher exact tests. The continuous data were compared between groups using the Mann–Whitney U and Wilcoxon non-parametric tests. The time-course data were analysed using the Kaplan–Meier method. Statistical significance was calculated using the log-rank test, and p values <0.05 were considered significant.
Results
Patient characteristics
A total of 94 patients with SpA were enrolled in the study, of whom 53 (56.4%) were men, with a mean (±SD) age of 50±11 years. Their demographic and clinical characteristics are shown in table 1. Of the 94 patients with SpA, 56 were analysed at 6 months (44 without ATI and 12 with ATI), 51 at 1 year (41 without ATI and 10 with ATI) and 56 at >4 years (44 without ATI and 12 with ATI). All of the patients received the standard regimen of 5 mg/kg IFX every 8 weeks; however, 18 (19%) of them needed more frequent infusions because the response obtained was inadequate.
Demographic characteristics of patients
Clinical response and association with serum IFX and ATI levels
ATI were detected in the serum samples of 24 (25.5%) patients, all of whom had undetectable serum trough IFX levels. In most of these patients, the antibodies appeared after the sixth infusion (median 44, IQR 24–55 weeks). In 17/24 (71%), the ATI occurred in the first year of IFX treatment, and in three (12.5%) patients, the antibodies were detected after 2 years (see online supplementary figure S1). We could not determine the exact moment of ATI development in four of the 24 patients because their initial samples were positive.
All patients had active disease at baseline, as indicated by a mean ASDAS of 3.08±1.31, with no differences in ASDAS values between the patients who subsequently did (2.94±0.72) or did not (3.14±1.46) develop ATI (p=0.534). The patients with ATI had significantly higher clinical activity (as measured by the ASDAS) at 6 months (2.55±0.89 vs 1.79±1.04, p=0.038), 1 year (1.95±0.67 vs 1.67±0.71, p=0.042) and >4 years (2.52±0.99 vs 1.53±0.81, p=0.024) of follow-up (figure 1A). The change in ASDAS (ΔASDAS) values was lower in the group of patients who developed antibodies at any time (0.48±0.73 vs 1.47±1.66, p=0.029 at 6 months; 0.81±1.20 vs 1.56±1.67, p=0.098 at 1 year; 0.45±0.82 vs 1.43±1.25 p=0.022 at >4 years) (figure 1B).The patients with ATI had less pronounced clinical improvement by ASDAS criteria at 6 months (13% vs 87%, p=0.077), 1 year (9.1% vs 90.9%, p=0.105) and > 4 years (8.7% vs 91.3%, p=0.05). During the study, 51 patients (54.3%) showed clinically significant improvement, only nine (17.6%) of whom developed ATI (p=0.047).
(A) Association between clinical activity, as measured by the ASDAS, and ATI development. ASDAS values (mean ± SD) at baseline, 6 months, 1 year and >4 years in the patients who developed ATI (□) and in those who did not develop ATI (■). (B) Association between clinical improvement, as measured by ASDAS (mean ± SD), and the absence (■) or presence (□) of ATI in the patients with SpA. ASDAS, Ankylosing Spondylitis Disease Activity Score; ATI, anti-infliximab antibody; SpA, spondyloarthritis.
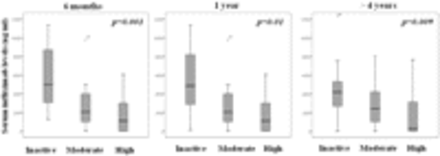
The patients with inactive disease came mainly from the group with no detectable ATI (43.5% without ATI vs 0% with ATI, p=0.001 at 6 months; 31.4% without ATI vs 12.5% with ATI, p=0.091 at 1 year; and 48.7% without ATI vs 9% with ATI, p=0.001 at >4 years). Patients with inactive disease had higher IFX levels (median, IQR) than those with active disease (inactive: 4992, 2976–8768 ng/ml vs moderate: 2048, 840–4112 ng/ml vs high: 1104, 0–3150 ng/ml, p=0.001 at 6 months; inactive: 4128, 2768–7824 ng/ml vs moderate: 2336, 852–3568 ng/ml vs high: 1328, 0–3366 ng/ml, p=0.010 at 1 year; inactive: 4192, 2872–5344 ng/ml vs moderate: 2432, 636–4288 ng/ml vs high: 308, 0–3296 ng/ml, p=0.009 at >4 years) (figure 2). Consequently, the ATI levels (median, IQR) were lower in the patients in remission (0, 0–0 AU/ml vs 0, 0–672 AU/ml, p=0.006 at 6 months; 0, 0–0 AU/ml vs 0, 0–447 AU/ml, p=0.074 at 1 year; 0, 0–0 AU/ml vs 0, 0–577 AU/ml, p=0.018 at >4 years).
The association between clinical activity as measured by ASDAS (inactive, moderate and high/very high activity) and infliximab levels at 6 months, 1 year and >4 years. The data are presented as interquartile ranges (75th centile, upper edge; 25th centile, lower edge; and 50th centile, midline of the box). ASDAS, Ankylosing Spondylitis Disease Activity Score.
Modulating ATI levels by shortening the intervals between infusions
In 18 (19%) of the 94 patients, the time between infusions was shortened owing to insufficient responses. This change was required more frequently in the patients with ATI (8/24 (33.3%) vs 10/70 (14.3%); p=0.044, respectively). A clinically significant improvement was seen more often in the patients without ATI (8/10 (80%) vs 2/8 (25%), p=0.031, respectively). In four patients (50%), the ATI levels were reduced to negative levels after shortening the infusion intervals. Moreover, the ATI levels of these four patients before the more frequent infusions were lower than those of the patients in whom ATI remained detectable after shortening the interval between infusions (median, IQR) (121.5, 68.5-191.0 vs 30000, 1325-41625; p=0.014, respectively).
Survival analysis for the IFX treatment
Twenty-seven (28.7%) of the 94 patients interrupted their IFX treatment, mainly owing to an insufficient response or the development of infusion-related reactions, with a median survival time of 6.99 years (95% CI 6.28 to 7.7). A larger fraction of the patients with ATI discontinued their IFX treatment (18/24 (75%) vs 9/70 (12.8%); p<0.001). The median IFX survival time was 4.25 years (95% CI 3.06 to 5.43) in the patients with ATI versus 8.19 years (95% CI 7.54 to 8.85) in the patients without ATI (p<0.001) (figure 3). The patients with ATI who did not discontinue the biological treatment had significantly lower antibody levels (0, 0–0 AU/ml vs 577, 0–5887 AU/ml; p=0.005) than did the patients who discontinued.
The infliximab survival curve in the patients with spondyloarthritis with/without ATI. Mean drug survival was shorter in the patients with ATI than in those without ATI (4.25 years, 95% CI 3.06 to 5.43 vs 8.19 years, 95% CI 7.54 to 8.85; p=0.001).
Relationship between ATI and infusion-related reactions
Infusion-related reactions were seen in 11 of 94 patients, most of whom had detectable ATI (8 (72.7%) vs 3 (27.3%), p=0.001). The ATI levels (median, IQR) at the times of the infusion reactions were significantly higher in the patients who developed infusion reactions (12 931, 853–82 437 AU/ml vs 2454, 449–7718 AU/ml; p=0.028) (figure 4).
The maximum anti-infliximab antibody (ATI) levels in the patients who developed or did not develop infusion-related reactions. The data are presented as interquartile ranges (75th centile, upper edge of the box; 25th centile, lower edge of the box; 50th centile, midline of the box).
Influence of combined treatment with MTX on ATI presence
A total of 47 out of 94 (50%) patients received concomitant MTX treatment (mean ± SD) (15±4.96 mg/weekly) sometime during the study, but only 36/94 (38%) patients were taking MTX before starting anti-TNF treatment. ATI were detected before starting MTX in nine of the remaining 11 patients; therefore, they were included in the group not taking MTX for the purposes of the statistical analysis. ATI developed more frequently in the patients not taking MTX (20/58 (34.5%) of those not taking MTX vs 4/36 (11.1%) of those taking MTX, p=0.011). In the four patients taking MTX who developed ATI, the appearance of antidrug antibodies occurred later (70.83±62.2 weeks in patients receiving IFX+MTX vs 36.50±16.58 weeks in patients receiving IFX alone, p=0.148). Moreover, the maximum IFX levels (mean ± SD) tended to be higher in the patients with concomitant MTX treatment (4548.26±3832.30 vs 3484.97±3018.47, respectively, p=0.147).
Discussion
This study shows that ATI formation in patients with SpA occurs most often after 6 months of anti-TNF treatment, although it can occur as long as 2 years after beginning IFX. The appearance of ATI is associated with a diminished clinical response, the development of infusion-related reactions, and a higher likelihood of needing more frequent infusions and discontinuing treatment. Concomitant MTX treatment also protects against the formation of ATI.
All biological drugs can induce an immune response, with the structure of anti-TNF agents being a decisive factor in their immunogenicity. The percentage of patients who develop ATI varies among different chronic inflammatory diseases. ATI have been seen in 12–44% of patients with RA,26,–,32 in 6–55% of patients with Crohn's disease33,–,37 and in up to 29% of patients with AS.18 Studies have demonstrated that chimeric drugs, which have murine components, have a greater likelihood of inducing antidrug antibody development than do fully human antibodies.38 The question that remains is why all patients treated with anti-TNF agents do not develop antidrug antibodies. Immunogenicity seems to be the result of several factors associated with the treatment, the patient and external factors,35 ,39 ,40 such as dose, treatment formulation, assay technology, contaminants in the host cells, genetic background and concomitant treatment with immunosuppressive drugs.35 ,40,–,43
In our group's previous study of patients with RA treated with IFX,24 we found that ATI development occurs most frequently within the first 4 months (median 16 weeks; IQR 14–79) of treatment; whereas in patients with SpA, it usually occurs after 6 months. Patients with SpA are treated with higher IFX doses (5 mg/kg) from the onset of treatment; therefore, greater antibody production would be needed to neutralise the higher IFX concentration,24 ,44 and immunotolerance is more likely to develop.41 This observation suggests that the frequency of ATI formation may have been underestimated in previous studies of immunogenicity in SpA with short observation times.18 ,20,–,22 Another factor that may underestimate the immunogenicity of IFX is the type of assay used to detect ATI (radioimmunoassay or ELISA) and the timing of sample extraction.
It is well known that the appearance of antibodies against a drug has a negative effect on the clinical response to that drug.18 ,24 ,45 ,46 A clinically significant improvement was not seen in 15 (62.5%) of the 24 patients with ATI. Moreover, the patients with ATI had significantly greater clinical activity at all the observation time points, and only a minority of the patients with ATI achieved remission. In previous publications on patients with SpA, the appearance of antidrug antibodies has also been associated with a poor clinical response.18 ,20 ,45 Conversely, more patients with measurable serum trough IFX levels reported clinical improvement during the study, and higher IFX levels were associated with inactive disease at any time point studied. Consistent with our results, de Vries et al observed a positive correlation between clinical response and IFX levels in 38 patients with AS treated with IFX for 54 weeks.18 Similar findings have also been described with other biological agents, such as adalimumab45 and etanercept.47 As we have shown, IFX levels are closely correlated with clinical activity, and the presence of ATI should be suspected when low or undetectable IFX levels are found.
In 19% of the patients, the interval between infusions was shortened to 6–7 weeks because of an insufficient response. Most of the patients without ATI (80%) achieved a significant clinical improvement with the more frequent infusions. Four out of eight patients with ATI achieved undetectable ATI after more frequent infusions and two of these patients showed significant clinical improvement with the adjusted therapeutic regimen. This result is probably due to the higher free serum IFX levels attained. Higher serum levels would neutralise ATI after increasing the dose or shortening the interval between infusions as previously described.44 Other authors have reported a lack of clinical improvement in patients with AS treated with IFX when the drug infusion interval was modified in patients with ATI2. We suggest that any modification in the therapeutic regimen (a dose increase or more frequent infusions) that is not followed by neutralisation of the antibody levels may not provide clinical benefits. Therefore, monitoring ATI levels should play an important role in avoiding the continuation of ineffective treatment.
The appearance of infusion-related reactions is one of the most important side effects of IFX treatment in different inflammatory diseases, as has been widely described.22 ,24 ,29 ,48 In our cohort, most of the patients with infusion-related reactions had high ATI levels (72.7%), which is similar to the results of our previous study in RA patients treated with IFX.24 Ducourau et al also found a strong correlation between ATI development and the appearance of an infusion-related reaction.22
Several studies have suggested that patients with RA treated with combined treatment (IFX and MTX) have less frequent ATI development22 ,29 or that they develop lower ATI levels.24 ,32 In our study, we found a significantly reduced rate of ATI development in the patients receiving combined treatment, as has been recently reported by others.22 Moreover, we found that the appearance of ATI was delayed in the patients receiving MTX. These findings may explain why, in some recent studies,21 ,49 no influence of MTX on IFX exposure has been found among patients with AS. The authors measured IFX concentrations for no longer than 1821 or 2250 weeks, at which point, in our experience, most patients with SpA will not have developed ATI. Contrary to the reports of some previous studies,21 ,49 we found that the maximum IFX levels tended to be higher in the patients with concomitant MTX treatment. This finding suggests that combined MTX treatment has a significant anti-immunogenic effect in preventing ATI development,51 which results in higher serum IFX levels.
In conclusion, ATI development is associated with a poor clinical response, the discontinuation of treatment and an increased incidence of adverse effects. Long-term follow-up demonstrates that ATI may form at any time, resulting in secondary inefficacy. Therapeutic regimen modulation seems to be more useful for achieving a clinical improvement in patients without ATI. Our results suggest that combined MTX treatment is useful for avoiding ATI development.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Web Only Data - This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
-
Funding This study was funded by unrestricted medical grant from Pfizer Laboratories of Spain and Proteomika SL. This work was also supported by Ministerio de Ciencia e Innovación grant SAF 2009-07100, and by RETICS Program, RD08/0075 (RIER) from “Instituto de Salud Carlos III” (ISCIII).
-
Competing interests AB has received fees from Roche, Schering-Plough, Wyeth, Abbott, BMS and USB. EM-M is a consultant and a member of speakers' bureaus for Pfizer, MSD, UCB and Abbott. CP, DP-S, GB and LN have received speaker honoraria from Pfizer. All other authors have declared no conflicts of interest.
-
Patient Consent Obtained.
-
Provenance and peer review Not commissioned; externally peer reviewed.