Article Text
Abstract
Background: The pathogenesis of the early stages of hand osteoarthritis is poorly understood, but recent high-resolution magnetic resonance imaging (hrMRI) studies suggest that the joint ligaments have a major role in the phenotypic expression of the disease.
Objective: To combine hrMRI and cadaveric histological studies to better understand the mechanisms of damage, and especially the role of joint ligaments and tendons in disease expression.
Methods: hrMRI was carried out in the distal interphalangeal (DIP) and proximal interphalangeal (PIP) joints in 20 patients with osteoarthritis,with a disease duration ⩽12 months. Histological sections of the DIP and PIP joints were obtained from three dissecting-room specimens for comparative analysis.
Results: The collateral ligaments influenced the location of both hrMRI-determined bone oedema and bone erosion in early osteoarthritis. These changes were best understood in relation to the enthesis organ concept, whereby the interaction between ligament fibrocartilages leads to bone disease. Normal ligaments were commonly associated with microdamage at insertions corresponding to ligament thickening noted in early osteoarthritis. The ligaments also influenced the location of node formation in early osteoarthritis. The DIP extensor tendon insertions were associated with the development of a neoarticular surface.
Conclusions: Small-joint collateral ligaments and tendons have a central role in the early stages of hand osteoarthritis, and determine the early expression of both the soft tissue and bony changes in disease.
- DIP, distal interphalangeal
- hrMRI, high-resolution magnetic resonance imaging
- MRI, magnetic resonance imaging
- PIP, proximal interphalangeal
Statistics from Altmetric.com
- DIP, distal interphalangeal
- hrMRI, high-resolution magnetic resonance imaging
- MRI, magnetic resonance imaging
- PIP, proximal interphalangeal
The pathogenesis of the early stages of osteoarthritis is poorly understood, but considerable emphasis has been placed on the role of cartilage and subchondral bone in the disease process.1–3 However, it is difficult to study the early phases of osteoarthritis in humans because clinical and radiographic evidence for joint degeneration is often marked at disease presentation.4 Furthermore, the existing imaging modalities cannot visualise in adequate detail the entire “joint organ”, especially constituent ligaments, tendons, capsules and entheses, owing to the inherently low signal of these structures and the relatively low spatial resolution of conventional magnetic resonance imaging (MRI).
To overcome such difficulties, we used high-resolution MRI (hrMRI) using surface coils adapted for clinical scanners, with an in-plane resolution of 80–100 μm.5 We evaluated early hand osteoarthritis, in which the disease is readily recognised even where plain radiographs are normal, and have shown that the most striking abnormalities were in the collateral ligaments and capsules of the proximal interphalangeal (PIP) and distal interphalangeal (DIP) joints, rather than in the articular cartilage or subchondral bone.5 We noticed an apparent relationship among the ligaments, bone erosion and bone oedema in small joints. We also noted that ligaments were abnormal in normal joints adjacent to clinically involved joints and in older normals. The basis for these observations remains unclear. The purpose of this article was to combine hrMRI conducted on hands with clinical osteoarthritis, with histological studies from cadaveric tissue, to better understand the microanatomical basis for the changes noted in ligaments and tendons.
METHODS
This account is illustrated with hrMRI obtained from 20 patients with early hand osteoarthritis (mean age 57 years, (range 49–70) all women, mean disease duration 7.6 months (range 1–12)), in whom the duration of disease was ⩽12 months. These patients had clinically osteoarthritic and painful finger joints, for which other inflammatory arthritis, including rheumatoid and psoriatic arthritis, and gout have been excluded. This group of patients included those reported previously, but the current study focused on the collateral ligaments and tendons, and the associated changes.
The local ethics committee approved the study and all patients gave written informed consent. hrMRI was carried out using a 1.5-T Gyroscan ACS-NT scanner (Philips Medical Systems, Best, The Netherlands) with a 23-mm diameter microscopy surface coil with a displayed pixel dimension of 80–100 μm as previously described.5
It is impossible to have corresponding histological sections of the above group of imaged joints. However, to aid the interpretation of the MRI scans, histological sections of the DIP and PIP joints from the index finger of one hand in each of three elderly dissecting-room cadavers (ages 73–77 years) were also examined, together with radiographs of the same specimens taken on a Faxitron Specimen Radiography System (Model MX-20, Wheeling, Illinois, USA). These fingers, although not clinically obviously osteoarthritic, may show some osteoarthritic changes in the joint owing to their age; we have previously shown on hrMRI that clinically normal finger joints from healthy volunteers do show subtle ligamentous changes.5 The bodies had been donated to Cardiff University for anatomical investigation under the provision of the 1984 Anatomy Act and the 1961 Human Tissues Act. All material was formalin fixed, decalcified in 5% nitric acid, processed for routine histology, embedded at 58°C in paraffin wax, sectioned in the sagittal or coronal planes at 8 μm and stained with Masson’s trichrome.
RESULTS
Ligament abnormalities
As previously noted,5 the collateral ligaments of the DIP and PIP joints were universally abnormal, with abnormalities ranging from thickening to frank disruption, even where articular cartilage seemed to be relatively unchanged. We noted that ligament-related inflammation in acute osteoarthritis was associated with florid gadolinium enhancement in the adjacent extracapsular tissues. The basis for these changes was explored using histological studies.
Entheseal relationship of bone oedema
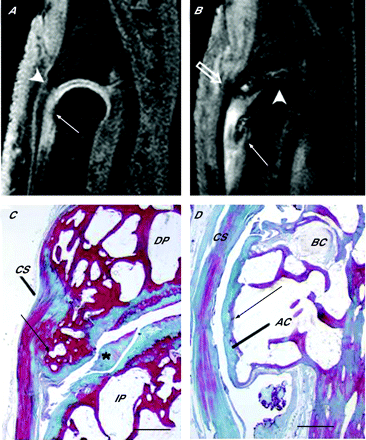
Prominent entheseal disease was a common feature of the pan-ligamentous changes in small-joint osteoarthritis, ranging from changes at the soft-tissue side of the enthesis to changes in the bone (fig 1). Abnormalities were more common in the collateral ligament entheses, but were also seen in the enthesis of the extensor tendon. Bone oedema at the collateral ligament origins and of the adjacent bone (55% of early osteoarthritis) was an interesting feature of early osteoarthritis. This pattern of bone oedema extended from the ligament origin to the subchondral regions and was evident even where articular cartilage was well preserved (fig 1B,C). We believe that this pattern of bone oedema is explained by the enthesis organ concept,6 whereby (fibro)cartilage is present at the attachment of the collateral ligament and in adjacent regions as well (fig 2C). To promote uniaxial movements of the interphalangeal joints, the proximal end of the collateral ligaments is attached at some distance from the joint cavity (fig 2C). Consequently, the region of the ligament immediately adjacent to this enthesis is pressed against the side of the phalanges when abduction or adduction of these hinge joints is passively resisted (fig 2). Owing to the mutual pressure of ligament against bone, the adjacent surfaces are (fibro)cartilaginous and the whole region contributes to the formation of an “enthesis organ”.6 Thus, there is a periosteal fibrocartilage on the surface of the bone and a sesamoid fibrocartilage at the deep surface of the ligament (fig 2C,D). Both show evidence of degeneration in elderly dissecting-room cadavers (fig 2C,D). The hrMRI scans suggest that bone oedema on the proximal phalanges seems to emanate from the region of this enthesis organ, and in some cases extends to the subchondral bone beneath the adjacent articular cartilage (fig 1A), in a manner similar to the pattern of bone oedema seen in enthesis organs at other sites.7 The extensive nature of bone oedema emanating from these sites in the presence of normal cartilage may provide a conceptual link for how small-joint bone changes related to the ligament could have a detrimental effect on cartilage, thus linking ligament disease as a potential inciting factor in bone and cartilage degenerative change.
Entheseal-related bone changes shown by (A) an osteoarthritic distal interphalangeal (DIP) joint scanned using T2-weighted fat-suppressed coronal sequence (B) a T1-weighted coronal image of a different DIP joint and (C) the corresponding post-gadolinium image showing a bone erosion that enhances after contrast (open arrows). (A) Thickened ligaments, which were degenerative bilaterally with fraying at the enthesis, can be seen (arrowheads). Prominent bone oedema is seen on the proximal phalange (arrow), which has high signal. This oedema has a relationship with the ligament that articulates with the adjacent bone as shown in fig 2. (B, C) As in the case of bone oedema, erosions are commonly related to the collateral ligaments being present adjacent to the ligament (arrow heads). This shows how the position of the collateral ligaments influences the expression of periarticular bone oedema and bone erosion in hand osteoarthritis.
Radiological, osteological and histological images of the sites of attachment of the collateral ligaments of the distal interphalangeal (DIP) joints, showing the enthesis organ concept in relation to small-joint osteoarthritis and how ligaments could effect bone oedema and erosion at these sites. (A) An anteroposterior radiograph of a joint from an elderly dissecting-room cadaver printed to visualise the soft-tissue relief of a collateral ligament (CL). The relatively proximal site of attachment of the ligament to the intermediate phalange (IP) means that the ligament presses against the lateral aspect of the bone in the region indicated by the arrow. BC, bone cyst; DP, distal phalange. (B) A low-power view of a coronal histological section of a joint to show the area illustrated radiologically in (A). The thick collateral ligament presses against articular cartilage on the side of the intermediate phalange in the region of the arrow. (C) A collateral ligament leaves a smooth, well-circumscribed marking (dotted line), devoid of vascular foraminae, on the intermediate phalange, which indicates the site of its enthesis (E). Consequently, in crossing the joint, the ligament presses against the side of the phalange in the region of the arrows. This area of bone is covered by a periosteal fibrocartilage that is continuous with the articular cartilage itself and is shown histologically in (B, C). The presence of fibrocartilage indicates that the bone is considerably compressed during ligament function at these sites. (D) A section in the coronal plane, through a collateral ligament of the proximal interphalangeal (PIP) joint in an elderly dissecting-room cadaver. The head of the proximal phalange (PP) is covered with a periosteal fibrocartilage (PF) that articulates with the CL—the two are separated by the joint cavity (*) that extends around the side of the bone. The anatomical location of the bone cyst (BC) is underneath the periosteal fibrocartilage. Scale bar, 100 µm. (E) A higher power view of (D) to show the evidence of osteoarthritic degenerative change, fissuring and cell clustering (arrows), both in the periosteal fibrocartilage and in the collateral ligament. This supports the concept that the magnetic resonance imaging ligamentous abnormalities are caused by degenerative changes and are a key part of cartilage-related disease in osteoarthritis. Scale bar, 100 µm.
Entheseal relationship of joint erosion
Although erosions are well recognised radiographically in osteoarthritis, they are nevertheless considered to be uncommon.8 The pathogenesis of erosive hand osteoarthritis is poorly understood, and its relationship to non-erosive osteoarthritis is unclear. hrMRI showed that erosions were quite common (65% of early osteoarthritis), which reflects the ability of hrMRI to detect small cortical breaks (fig 1B,C). Two patterns of erosions were noted:
-
Periarticular erosions, which were sometimes related to the collateral ligaments (fig 1B,C).
-
“Seagull-wing erosions,”9,10 which were related to severe cartilage loss and osteophyte formation (fig 3A,B).
(A, B) A plain radiograph and magnetic resonance imaging scan of the same distal interphalangeal joint of a 61-year-old woman showing a “seagull-wing” pattern of bone erosion. This image suggests that seagull-wing erosions have a different mechanism that is not directly linked to the position of ligaments. Instead, the erosions appear at sites of prominent cartilage loss with bone remodelling, with bone loss on the distal part of the joint and bone growth proximally that abuts distally, giving the radiographic erosion appearance.
The complex structure of the collateral ligaments provides a biomechanical mechanism, whereby ligament-related disease may affect the phenotypic expression of erosions in a manner similar to that of bone oedema described earlier. In osteoarthritis, as in rheumatoid arthritis, bone oedema and erosion probably form part of the same spectrum.11
The classical seagull-wing type central erosion in osteoarthritis has a different mechanism and seems to relate to severe cartilage loss with distal-joint subchondral bone collapse and proximal central osteophyte abutting on the contralateral bony surface.9
Ligament changes in osteoarthritis and with age
Ligament thickening and signal changes were conspicuous in osteoarthritis and in older normal subjects as previously described. The histological sections from normal elderly dissecting-room cadavers show considerable degenerative changes in the ligaments near their origins (fig 2C,D). These changes explain, at least partly, the abnormalities evident in early hand osteoarthritis (fig 1).
Entheseal relationship of Heberden’s and Bouchard’s nodes
It has been suggested that Heberden’s and Bouchard’s nodes are similar to Baker’s cysts in the knee, and that inflammatory tissue bulges through points of intrinsic weakness in the joint capsules, leading to the formation of acute nodes, with subsequent ossification leading to bony nodes.12 As previously reported,5 in early osteoarthritis, we noticed inflammatory tissue bulging through the joint capsules at characteristic points of weakness between the collateral ligaments and the extensor tendon, just as predicted (fig 4A). In chronic osteoarthritis, we noted that the nodes formed at the same site (fig 4B). The restraining effect of the collateral ligaments and the extensor tendons thus seemed to determine the most characteristic clinical feature of generalised nodal osteoarthritis.
Formation of nodal osteoarthritis seen on axial magnetic resonance imaging scans of the proximal interphalangeal joints with early osteoarthritis in a patient with (A) symptom duration 5 weeks and (B) a more advanced early osteoarthritis joint. The joint in (A) scanned with gadolinium contrast was swollen with soft-tissue swelling bulging dorsally, pushing the extensor tendon slips apart (*). Marked enhancement was seen, indicating active inflammation in the joint. An osteophyte was seen in the joint in (B) in the same area where the soft tissue tended to bulge through (arrow). This supports the hypothesis that the clinical features of nodal osteoarthritis, to some degree, mirror Baker’s cyst formation in the knee joint.12
Tendon abnormalities
It was noted that the extensor tendons over both the DIP and PIP joints were abnormal in most cases (80%) of early osteoarthritis, but that disease was generally less marked than in the ligaments. The changes comprised soft-tissue abnormalities, with thickening and degeneration in the tendon, identical to changes observed in the ligaments (fig 5A,B). Interestingly, these changes were not seen at the insertion of the deep flexor tendon on the volar side of the joint. The bone at the distal phalangeal insertion of the extensor tendon was often associated with osteophyte formation (fig 5).
(A, B) Sagittal gradient echo water selective excitation high-resolution magnetic resonance imaging scans of two early osteoarthritic distal interphalangeal (DIP) joints. (C, D) Histological sections cut in the sagittal plane through the DIP joint of an elderly dissecting-room cadaver. The extensor tendon is thickened in (A) (arrowhead) despite a relatively well-preserved joint space. A small osteophyte was seen dorsally on the head of the intermediate phalange (arrow). An enthesophyte is present at the insertion in (B) (open arrow), with a corresponding hook-like osteophyte proximally (arrow). This joint also shows the typical pattern of cartilage loss on the volar aspect (arrowhead). The congruent appearances of the osteophyte and enthesophyte closely relate to the joint capsule shape. (C) Heberden’s node (arrow) at the attachment site of the central slip (CS) of the extensor tendon to the base of the distal phalange (DP). Articular cartilage that has formed on its surface is continuous with that on the rest of the bone. Degenerative changes (*) in the articular cartilage cover the head of the intermediate phalange (IP). (D) A bony spur (arrow) extending from the dorsal surface of the intermediate phalange under the central slip of the extensor tendon. It is covered with articular cartilage (AC) and has a small bone cyst in it. The bony spur is similar to that shown in (B). Scale bar, 1 mm.
Other changes—patterns of joint remodelling
Although cartilage loss was quite extensive in some patients with chronic osteoarthritis, the most consistent site of severe loss was on the volar surface of the joint (fig 5B). Where volar cartilage loss was pronounced, we noted that this was often associated with compensatory osteophyte formation on the dorsal aspect of the joint, both distally and proximally (fig 5B), and that cartilage formation occurred on the surface of osteophytes next to the joint cavity. This suggested that with the complete loss of cartilage at one site, the osteophytes were contributing to a “neoarticular” surface as part of the adaptive response to osteoarthritis. This is what we observed in cadaveric specimens with corresponding dorsal osteophytes lined by articular cartilage (fig 5C); the decompensated joint remodelling is evident at disease onset.
DISCUSSION
Our previous study highlighted the hitherto unappreciated role of ligaments in the phenotypic expression of changes in the soft tissue and bone in patients with generalised non-traumatic osteoarthritis. These findings in the early stages of human disease parallel recent observations on animal models, where spontaneous non-traumatic osteoarthritis started in joint ligaments in the knee13 and where ligamentous damage was a prerequisite for joint degeneration in osteoarthritis.14 The present clinicopathological correlates highlight how the joint ligaments, tendons and entheses seem to influence the phenotypic expression of all the bony and soft-tissue features of generalised nodal osteoarthritis.
It was previously noted in older healthy people, that ligament changes (especially thickening) were common, and that such changes were sometimes identical to those seen in clinically and radiographically normal joints, adjacent to diseased osteoarthritis joints. These observations are of particular interest because osteoarthritis is a disease of ageing. Although the hrMRI observations do not in any way imply cause and effect, they do indicate that normal ageing, osteoarthritis and ligament degeneration may be inter-related, whereas there is no obvious relationship with articular cartilage, at least in the small joints of the hand.
The limitations of this study include the limitations of MRI for defining the nature of the abnormality in the ligaments and the fact that the cadaveric tissue was not matched for MRI scans. However, this is because it would be difficult to carry out MRI on cadaveric tissue, both for ethical reasons and because of the possibility of sample desiccation, and it was obviously not possible to obtain tissue directly from joints of the study participants.
Hand osteoarthritis has a genetic basis,15,16 and is strongly associated with osteoarthritis at other sites including the knee and the hip,17 but the limitations of spatial resolution of MRI for imaging large joints presently make it difficult to ascertain the role of ligaments in the early stages of hip and knee disease. Our observations also link the concept of ageing and osteoarthritis to abnormalities of the joint ligaments. On the basis of these data from the hand joints in patients with hereditary osteoarthritis, we believe that the role of ligaments as an initiating or perpetuating factor in the pathogenesis of non-traumatic knee and hip disease merits attention. The damage evident in the small-joint collateral ligaments raises the possibility that this is the principal site of wear and tear in early disease.
Acknowledgments
We thank all who referred patients for the study. We also thank all the staff at the Leeds General Infirmary MRI Unit, in particular Mr David Shelley, for their services with regard to the study; and T Barrett, S Redman and Koji Hayashi for help with histological sectioning.
REFERENCES
Footnotes
-
Published Online First 20 April 2006
-
Funding: This study was supported by the Medical Research Council, UK, and Action Medical Research and Search.
-
Competing interests: None.