Key Points
-
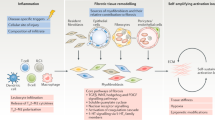
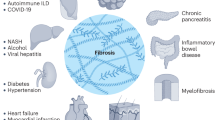
Fibrosis is characterized by excessive accumulation of connective tissue components in organs or tissues, which is caused by deregulated wound-healing processes in response to chronic tissue injury and/or inflammation
-
Chronic tissue injury and inflammation—hallmarks of rheumatic diseases—are crucial in activating the tissue repair mechanisms that result in fibrosis in systemic sclerosis (SSc)
-
Fibrosis in SSc is typically characterized by prolonged and/or exaggerated activation of fibroblasts, a key feature of which is the differentiation of fibroblasts into myofibroblasts
-
Endoplasmic reticulum stress has been hypothesized to contribute to the initiation of fibrotic tissue remodelling in rheumatic diseases, and mechanical cues have a crucial role in determining fibroblast activation in fibrosis
-
Mechanotransduction, the capability of cells to transform mechanical cues into biochemical signals, has also been implicated in fibrotic mechanisms
-
Therapies targeting either persistent fibroblast activation (such as aberrant immune responses), or the chemical and mechanical stimuli that drive myofibroblast differentiation, could have the potential to alleviate the symptoms of SSc
Abstract
Fibrosis is a pathological process characterized by excessive accumulation of connective tissue components in an organ or tissue. Fibrosis is produced by deregulated wound healing in response to chronic tissue injury or chronic inflammation, the hallmarks of rheumatic diseases. Progressive fibrosis, which distorts tissue architecture and results in progressive loss of organ function, is now recognized to be one of the major causes of morbidity and mortality in individuals with one of the most lethal rheumatic disease, systemic sclerosis (SSc). In this Review, we discuss the pathological role of fibrosis in SSc. We discuss the involvement of endothelium and pericyte activation, aberrant immune responses, endoplasmic reticulum stress and chronic tissue injury in the initiation of fibrosis in SSc. We then discuss fibroblast activation and myofibroblast differentiation that occurs in response to these initiating processes and is responsible for excessive accumulation of extracellular matrix. Finally, we discuss the chemical and mechanical signals that drive fibroblast activation and myofibroblast differentiation, which could serve as targets for new therapies for fibrosis in SSc.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Guillevin, L. et al. Functional impairment of systemic scleroderma patients with digital ulcerations: results from the DUO Registry. Clin. Exp. Rheumatol. 31, 71–80 (2013).
Gelber, A. C. et al. Race and association with disease manifestations and mortality in scleroderma: a 20-year experience at the Johns Hopkins Scleroderma Center and review of the literature. Medicine (Baltimore) 92, 191–205 (2013).
Nikpour, M. & Baron, M. Mortality in systemic sclerosis: lessons learned from population-based and observational cohort studies. Curr. Opin. Rheumatol. 26, 131–137 (2014).
Bhattacharyya, S., Wei, J. & Varga, J. Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat. Rev. Rheumatol. 8, 42–54 (2012).
Trojanowska, M. Cellular and molecular aspects of vascular dysfunction in systemic sclerosis. Nat. Rev. Rheumatol. 6, 453–460 (2010).
Hachulla, E. & Launay, D. Diagnosis and classification of systemic sclerosis. Clin. Rev. Allergy Immunol. 40, 78–83 (2011).
Rudnicka, L. et al. Elevated expression of type VII collagen in the skin of patients with systemic sclerosis. Regulation by transforming growth factor-beta. J. Clin. Invest. 93, 1709–1715 (1994).
Makino, K. et al. The downregulation of microRNA let-7a contributes to the excessive expression of type I collagen in systemic and localized scleroderma. J. Immunol. 190, 3905–3915 (2013).
Maurer, B. et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 62, 1733–1743 (2010).
Honda, N. et al. miR-150 down-regulation contributes to the constitutive type I collagen overexpression in scleroderma dermal fibroblasts via the induction of integrin β3. Am. J. Pathol. 182, 206–216 (2013).
Chanoki, M. et al. Increased expression of lysyl oxidase in skin with scleroderma. Br. J. Dermatol. 133, 710–715 (1995).
Fleming, J. N. et al. Cutaneous chronic graft-versus-host disease does not have the abnormal endothelial phenotype or vascular rarefaction characteristic of systemic sclerosis. PLoS ONE 4, e6203 (2009).
Manetti, M. et al. Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis. J. Cell. Mol. Med. 17, 482–496 (2013).
Ceafalan, L., Gherghiceanu, M., Popescu, L. M. & Simionescu, O. Telocytes in human skin—are they involved in skin regeneration? J. Cell. Mol. Med. 16, 1405–1420 (2012).
Bani, D., Formigli, L., Gherghiceanu, M. & Faussone-Pellegrini, M. S. Telocytes as supporting cells for myocardial tissue organization in developing and adult heart. J. Cell. Mol. Med. 14, 2531–2538 (2010).
Popescu, L. M., Gherghiceanu, M., Suciu, L. C., Manole, C. G. & Hinescu, M. E. Telocytes and putative stem cells in the lungs: electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 345, 391–403 (2011).
Milano, A. et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE 3, e2696 (2008).
Gardner, H. et al. Gene profiling of scleroderma skin reveals robust signatures of disease that are imperfectly reflected in the transcript profiles of explanted fibroblasts. Arthritis Rheum. 54, 1961–1973 (2006).
Guiducci, S., Giacomelli, R. & Cerinic, M. M. Vascular complications of scleroderma. Autoimmun. Rev. 6, 520–523 (2007).
Kuwana, M., Okazaki, Y., Yasuoka, H., Kawakami, Y. & Ikeda, Y. Defective vasculogenesis in systemic sclerosis. Lancet 364, 603–610 (2004).
Rabquer, B. J. & Koch, A. E. Angiogenesis and vasculopathy in systemic sclerosis: evolving concepts. Curr. Rheumatol. Rep. 14, 56–63 (2012).
Morgan-Rowe, L. et al. Thrombospondin 1 in hypoxia-conditioned media blocks the growth of human microvascular endothelial cells and is increased in systemic sclerosis tissues. Fibrogenesis Tissue Repair 4, 13 (2011).
Koch, A. E. et al. In situ expression of cytokines and cellular adhesion molecules in the skin of patients with systemic sclerosis. Their role in early and late disease. Pathobiology 61, 239–246 (1993).
Gruschwitz, M. S. & Vieth, G. Up-regulation of class II major histocompatibility complex and intercellular adhesion molecule 1 expression on scleroderma fibroblasts and endothelial cells by interferon-gamma and tumor necrosis factor alpha in the early disease stage. Arthritis Rheum. 40, 540–550 (1997).
Rajkumar, V. S., Sundberg, C., Abraham, D. J., Rubin, K. & Black, C. M. Activation of microvascular pericytes in autoimmune Raynaud's phenomenon and systemic sclerosis. Arthritis Rheum. 42, 930–941 (1999).
Rajkumar, V. S. et al. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res. Ther. 7, R1113–R1123 (2005).
Lin, S. L., Kisseleva, T., Brenner, D. A. & Duffield, J. S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 173, 1617–1627 (2008).
Amodio, G. & Gregori, S. Dendritic cells a double-edge sword in autoimmune responses. Front. Immunol. 3, 233 (2012).
Nace, G., Evankovich, J., Eid, R. & Tsung, A. Dendritic cells and damage-associated molecular patterns: endogenous danger signals linking innate and adaptive immunity. J. Innate Immun. 4, 6–15 (2012).
Lu, T. T. Dendritic cells: novel players in fibrosis and scleroderma. Curr. Rheumatol. Rep. 14, 30–38 (2012).
Granucci, F., Zanoni, I. & Ricciardi-Castagnoli, P. Central role of dendritic cells in the regulation and deregulation of immune responses. Cell. Mol. Life Sci. 65, 1683–1697 (2008).
van Bon, L., Cossu, M. & Radstake, T. R. An update on an immune system that goes awry in systemic sclerosis. Curr. Opin. Rheumatol 23, 505–510 (2011).
Sugiura, H. et al. Activation of Toll-like receptor 3 augments myofibroblast differentiation. Am. J. Respir. Cell. Mol. Biol. 40, 654–662 (2009).
Meneghin, A. et al. TLR9 is expressed in idiopathic interstitial pneumonia and its activation promotes in vitro myofibroblast differentiation. Histochem. Cell Biol. 130, 979–992 (2008).
Farina, G. A. et al. Poly(I:C) drives type I IFN- and TGFβ-mediated inflammation and dermal fibrosis simulating altered gene expression in systemic sclerosis. J. Invest. Dermatol. 130, 2583–2593 (2010).
van Lieshout, A. W. et al. Enhanced interleukin-10 production by dendritic cells upon stimulation with Toll-like receptor 4 agonists in systemic sclerosis that is possibly implicated in CCL18 secretion. Scand. J. Rheumatol. 38, 282–290 (2009).
Fineschi, S. et al. Antifibroblast antibodies in systemic sclerosis induce fibroblasts to produce profibrotic chemokines, with partial exploitation of Toll-like receptor 4. Arthritis Rheum. 58, 3913–3923 (2008).
Roumm, A. D. et al. Lymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlations. Arthritis Rheum. 27, 645–653 (1984).
Gustafsson, R., Tötterman, T. H., Klareskog, L. & Hällgren, R. Increase in activated T cells and reduction in suppressor inducer T cells in systemic sclerosis. Ann. Rheum. Dis. 49, 40–45 (2003).
Zuber, J. P. & Spertini, F. Immunological basis of systemic sclerosis. Rheumatology (Oxford) 45 (Suppl. 3), iii23–iii25 (2006).
Yukawa, S. et al. Involvement of mast cells in systemic sclerosis. Nihon Rinsho Meneki Gakkai Kaishi 33, 81–86 (2010).
Duncan, M. R. & Berman, B. Stimulation of collagen and glycosaminoglycan production in cultured human adult dermal fibroblasts by recombinant human interleukin 6. J. Invest. Dermatol. 97, 686–692 (1991).
Wynn, T. A. Fibrotic disease and the TH1/TH2 paradigm. Nat. Rev. Immunol. 4, 583–594 (2004).
Hasegawa, M., Fujimoto, M., Kikuchi, K. & Takehara, K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J. Rheumatol 24, 328–332 (1997).
Bhogal, R. K. & Bona, C. A. Regulatory effect of extracellular signal-regulated kinases (ERK) on type I collagen synthesis in human dermal fibroblasts stimulated by IL-4 and IL-13. Int. Rev. Immunol. 27, 472–496 (2008).
Jinnin, M., Ihn, H., Yamane, K. & Tamaki, K. Interleukin-13 stimulates the transcription of the human α2(I) collagen gene in human dermal fibroblasts. J. Biol. Chem. 279, 41783–41791 (2004).
Yamamoto, T. Autoimmune mechanisms of scleroderma and a role of oxidative stress. Self Nonself 2, 4–10 (2011).
Wen, F. Q. et al. Interleukin-4- and interleukin-13-enhanced transforming growth factor-β2 production in cultured human bronchial epithelial cells is attenuated by interferon-gamma. Am. J. Respir. Cell. Mol. Biol. 26, 484–490 (2002).
Feghali, C. A., Bost, K. L., Boulware, D. W. & Levy, L. S. Human recombinant interleukin-4 induces proliferation and interleukin-6 production by cultured human skin fibroblasts. Clin. Immunol. Immunopathol. 63, 182–187 (1992).
Lee, Y. W., Kuhn, H., Hennig, B., Neish, A. S. & Toborek, M. IL-4-induced oxidative stress upregulates VCAM-1 gene expression in human endothelial cells. J. Mol. Cell Cardiol. 33, 83–94 (2001).
Atamas, S. P. et al. Production of type 2 cytokines by CD8+ lung cells is associated with greater decline in pulmonary function in patients with systemic sclerosis. Arthritis Rheum. 42, 1168–1178 (1999).
Riccieri, V. et al. Interleukin-13 in systemic sclerosis: relationship to nailfold capillaroscopy abnormalities. Clin. Rheumatol. 22, 102–106 (2003).
Aliprantis, A. O. et al. Transcription factor T-bet regulates skin sclerosis through its function in innate immunity and via IL-13. Proc. Natl Acad. Sci. USA 104, 2827–2830 (2007).
Fuschiotti, P. Role of IL-13 in systemic sclerosis. Cytokine 56, 544–549 (2011).
Fuschiotti, P., Medsger, T. A., Jr & Morel, P. A. Effector CD8+ T cells in systemic sclerosis patients produce abnormally high levels of interleukin-13 associated with increased skin fibrosis. Arthritis Rheum. 60, 1119–1128 (2009).
Rincon, M., Anguita, J., Nakamura, T., Fikrig, E. & Flavell, R. A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J. Exp. Med. 185, 461–469 (1997).
Hsieh, C. S., Heimberger, A. B., Gold, J. S., O'Garra, A. & Murphy, K. M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβ T-cell-receptor transgenic system. Proc. Natl Acad. Sci. USA 89, 6065–6069 (1992).
Fielding, C. A. et al. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity 40, 40–50 (2014).
Hasegawa, M. et al. Serum levels of interleukin 6 (IL-6), oncostatin M, soluble IL-6 receptor, and soluble gp130 in patients with systemic sclerosis. J. Rheumatol 25, 308–313 (1998).
Gallucci, R. M., Lee, E. G. & Tomasek, J. J. IL-6 modulates alpha-smooth muscle actin expression in dermal fibroblasts from IL-6-deficient mice. J. Invest. Dermatol. 126, 561–568 (2006).
Elhai, M. et al. Outcomes of patients with systemic sclerosis-associated polyarthritis and myopathy treated with tocilizumab or abatacept: a EUSTAR observational study. Ann. Rheum. Dis. 72, 1217–1220 (2013).
Shima, Y. et al. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology (Oxford) 49, 2408–2412 (2010).
Md Yusof, M. Y. & Emery, P. Targeting interleukin-6 in rheumatoid arthritis. Drugs 73, 341–356 (2013).
Cepeda, E. J. & Reveille, J. D. Autoantibodies in systemic sclerosis and fibrosing syndromes: clinical indications and relevance. Curr. Opin. Rheumatol. 16, 723–732 (2004).
Sato, S., Hamaguchi, Y., Hasegawa, M. & Takehara, K. Clinical significance of anti-topoisomerase I antibody levels determined by ELISA in systemic sclerosis. Rheumatology (Oxford) 40, 1135–1140 (2001).
Baroni, S. S. et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N. Engl. J. Med. 354, 2667–2676 (2006).
Ronda, N. et al. Antifibroblast antibodies from systemic sclerosis patients are internalized by fibroblasts via a caveolin-linked pathway. Arthritis Rheum. 46, 1595–1601 (2002).
Tan, F. K. et al. Autoantibodies to fibrillin 1 in systemic sclerosis: ethnic differences in antigen recognition and lack of correlation with specific clinical features or HLA alleles. Arthritis Rheum. 43, 2464–2471 (2000).
Nishijima, C. et al. Autoantibody against matrix metalloproteinase-3 in patients with systemic sclerosis. Clin. Exp. Immunol. 138, 357–363 (2004).
Lenna, S. & Trojanowska, M. The role of endoplasmic reticulum stress and the unfolded protein response in fibrosis. Curr. Opin. Rheumatol. 24, 663–668 (2012).
Tanjore, H., Lawson, W. E. & Blackwell, T. S. Endoplasmic reticulum stress as a pro-fibrotic stimulus. Biochim. Biophys. Acta 1832, 940–947 (2013).
Bertolotti, A., Zhang, Y., Hendershot, L. M., Harding, H. P. & Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2, 326–332 (2000).
Moore, K. A. & Hollien, J. The unfolded protein response in secretory cell function. Annu. Rev. Genet. 46, 165–183 (2012).
Lenna, S. et al. Increased expression of endoplasmic reticulum stress and unfolded protein response genes in peripheral blood mononuclear cells from patients with limited cutaneous systemic sclerosis and pulmonary arterial hypertension. Arthritis Rheum. 65, 1357–1366 (2013).
Ding, W. X. & Yin, X. M. Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy 4, 141–150 (2008).
Ishida, Y. & Nagata, K. Autophagy eliminates a specific species of misfolded procollagen and plays a protective role in cell survival against ER stress. Autophagy 5, 1217–1219 (2009).
Castello-Cros, R. et al. Scleroderma-like properties of skin from caveolin-1-deficient mice: implications for new treatment strategies in patients with fibrosis and systemic sclerosis. Cell Cycle 10, 2140–2150 (2011).
Picard, D. Heat-shock protein 90, a chaperone for folding and regulation. Cell. Mol. Life Sci. 59, 1640–1648 (2002).
Wrighton, K. H., Lin, X. & Feng, X. H. Critical regulation of TGFβ signaling by Hsp90. Proc. Natl Acad. Sci. USA 105, 9244–9249 (2008).
Tomcik, M. et al. Heat shock protein 90 (Hsp90) inhibition targets canonical TGF-β signalling to prevent fibrosis. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2012-203095.
Miyoshi, S., Yamazaki, S., Uchiumi, A. & Katagata, Y. The Hsp90 inhibitor 17-AAG represses calcium-induced cytokeratin 1 and 10 expression in HaCaT keratinocytes. FEBS Open Bio. 2, 47–50 (2012).
Noh, H. et al. Heat shock protein 90 inhibitor attenuates renal fibrosis through degradation of transforming growth factor-beta type II receptor. Lab. Invest. 92, 1583–1596 (2012).
Santiago, B., Galindo, M., Rivero, M. & Pablos, J. L. Decreased susceptibility to Fas-induced apoptosis of systemic sclerosis dermal fibroblasts. Arthritis Rheum. 44, 1667–1676 (2001).
Hinz, B., Dugina, V., Ballestrem, C., Wehrle-Haller, B. & Chaponnier, C. Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol. Biol. Cell 14, 2508–2519 (2003).
Jelaska, A., Arakawa, M., Broketa, G. & Korn, J. H. Heterogeneity of collagen synthesis in normal and systemic sclerosis skin fibroblasts. Increased proportion of high collagen-producing cells in systemic sclerosis fibroblasts. Arthritis Rheum. 39, 1338–1346 (1996).
Moulin, V. et al. Normal skin wound and hypertrophic scar myofibroblasts have differential responses to apoptotic inductors. J. Cell Physiol. 198, 350–358 (2004).
Hoyles, R. K. et al. An essential role for resident fibroblasts in experimental lung fibrosis is defined by lineage-specific deletion of high-affinity type II transforming growth factor beta receptor. Am. J. Respir. Crit. Care Med. 183, 249–261 (2011).
Vannella, K. M. et al. Cysteinyl leukotrienes are autocrine and paracrine regulators of fibrocyte function. J. Immunol. 179, 7883–7890 (2007).
Mehrad, B., Burdick, M. D. & Strieter, R. M. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 41, 1708–1718 (2009).
Distler, J. H. et al. Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum. 56, 4203–4215 (2007).
Humphreys, B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
Taura, K. et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology 51, 1027–1036 (2010).
Rock, J. R. et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl Acad. Sci. USA 108, E1475–E1483 (2011).
Zeisberg, E. M. et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 13, 952–961 (2007).
Biernacka, A., Dobaczewski, M. & Frangogiannis, N. G. TGF-β signaling in fibrosis. Growth Factors 29, 196–202 (2011).
Kitani, A. et al. Transforming growth factor (TGF)-β1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-β1-mediated fibrosis. J. Exp. Med. 198, 1179–1188 (2003).
Radstake, T. R. et al. Increased frequency and compromised function of T regulatory cells in systemic sclerosis (SSc) is related to a diminished CD69 and TGFβ expression. PLoS ONE 4, e5981 (2009).
Denton, C. P. et al. Recombinant human anti-transforming growth factor β1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 56, 323–333 (2007).
Tager, A. M. et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 14, 45–54 (2008).
Pradere, J. P. et al. LPA1 receptor activation promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 18, 3110–3118 (2007).
Sakai, N. et al. LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J. 27, 1830–1846 (2013).
Watanabe, N. et al. Plasma lysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity. Life Sci. 81, 1009–1015 (2007).
Watanabe, N. et al. Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. J. Clin. Gastroenterol. 41, 616–623 (2007).
Nikitopoulou, I. et al. Autotaxin expression from synovial fibroblasts is essential for the pathogenesis of modeled arthritis. J. Exp. Med. 209, 925–933 (2012).
Bourgoin, S. G. & Zhao, C. Autotaxin and lysophospholipids in rheumatoid arthritis. Curr. Opin. Investig. Drugs 11, 515–526 (2010).
Pattanaik, D. & Postlethwaite, A. E. A role for lysophosphatidic acid and sphingosine 1-phosphate in the pathogenesis of systemic sclerosis. Discov. Med. 10, 161–167 (2010).
Castelino, F. V. Lipids and eicosanoids in fibrosis: emerging targets for therapy. Curr. Opin. Rheumatol. 24, 649–655 (2012).
Sevastou, I., Kaffe, E., Mouratis, M. A. & Aidinis, V. Lysoglycerophospholipids in chronic inflammatory disorders: the PLA(2)/LPC and ATX/LPA axes. Biochim. Biophys. Acta 1831, 42–60 (2013).
Mutoh, T., Rivera, R. & Chun, J. Insights into the pharmacological relevance of lysophospholipid receptors. Br. J. Pharmacol. 165, 829–844 (2012).
Castelino, F. V. et al. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 63, 1405–1415 (2011).
Mazereeuw-Hautier, J. et al. Production of lysophosphatidic acid in blister fluid: involvement of a lysophospholipase D activity. J. Invest. Dermatol. 125, 421–427 (2005).
Tokumura, A. et al. Elevated serum levels of arachidonoyl-lysophosphatidic acid and sphingosine 1-phosphate in systemic sclerosis. Int. J. Med. Sci. 6, 168–176 (2009).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Memon, R. A. et al. Up-regulation of peroxisome proliferator-activated receptors (PPAR-α) and PPAR-γ messenger ribonucleic acid expression in the liver in murine obesity: troglitazone induces expression of PPAR-γ-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology 141, 4021–4031 (2000).
Ghosh, A. K. et al. Peroxisome proliferator-activated receptor-gamma abrogates Smad-dependent collagen stimulation by targeting the p300 transcriptional coactivator. FASEB J. 23, 2968–2977 (2009).
Wu, M. et al. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-γ. Am. J. Pathol. 174, 519–533 (2009).
Wei, J. et al. A synthetic PPAR-γ agonist triterpenoid ameliorates experimental fibrosis: PPAR-gamma-independent suppression of fibrotic responses. Ann. Rheum. Dis. 73, 446–454 (2013).
Patel, L. et al. Tumor suppressor and anti-inflammatory actions of PPARγ agonists are mediated via upregulation of PTEN. Curr. Biol. 11, 764–768 (2001).
Wei, J. et al. PPARγ downregulation by TGFβ in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLoS ONE 5, e13778 (2010).
Kapoor, M. et al. Loss of peroxisome proliferator-activated receptor γ in mouse fibroblasts results in increased susceptibility to bleomycin-induced skin fibrosis. Arthritis Rheum. 60, 2822–2829 (2009).
Chen, Y. et al. Inhibition of Notch signaling by a γ-secretase inhibitor attenuates hepatic fibrosis in rats. PLoS ONE 7, e46512 (2012).
Dees, C. et al. Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann. Rheum. Dis. 70, 1304–1310 (2011).
Dees, C. et al. Inhibition of Notch signaling prevents experimental fibrosis and induces regression of established fibrosis. Arthritis Rheum. 63, 1396–1404 (2011).
Wei, J. et al. Wnt/β-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 64, 2734–2745 (2012).
Wei, J. et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum. 63, 1707–1717 (2011).
Wang, P. S. et al. Thiazolidinediones downregulate Wnt/β-catenin signaling via multiple mechanisms in breast cancer cells. J. Surg. Res. 153, 210–216 (2009).
Shalhoub, V. et al. Calcification inhibitors and Wnt signaling proteins are implicated in bovine artery smooth muscle cell calcification in the presence of phosphate and vitamin D sterols. Calcif. Tissue Int. 79, 431–442 (2006).
Thorne, C. A. et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat. Chem. Biol. 6, 829–836 (2010).
Li, J. et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39, 754–766 (2006).
Reich, A., Meurer, M., Eckes, B., Friedrichs, J. & Muller, D. J. Surface morphology and mechanical properties of fibroblasts from scleroderma patients. J. Cell. Mol. Med. 13, 1644–1652 (2009).
Tschumperlin, D. J., Liu, F. & Tager, A. M. Biomechanical regulation of mesenchymal cell function. Curr. Opin. Rheumatol. 25, 92–100 (2013).
Liu, F. et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 190, 693–706 (2010).
Barry-Hamilton, V. et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 16, 1009–1017 (2010).
Kagan, H. M. & Li, W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J. Cell. Biochem. 88, 660–672 (2003).
Barker, H. E., Cox, T. R. & Erler, J. T. The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer 12, 540–552 (2012).
Meyringer, R. et al. Analysis of gene expression patterns in systemic sclerosis fibroblasts using RNA arbitrarily primed-polymerase chain reaction for differential display. J. Rheumatol. 34, 747–753 (2007).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
Geiger, B., Bershadsky, A., Pankov, R. & Yamada, K. M. Transmembrane crosstalk between the extracellular matrix—cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2, 793–805 (2001).
Shi-wen, X. et al. Focal adhesion kinase and reactive oxygen species contribute to the persistent fibrotic phenotype of lesional scleroderma fibroblasts. Rheumatology (Oxford) 51, 2146–2154 (2012).
Wong, V. W. et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat. Med. 18, 148–152 (2012).
Schultze, A. & Fiedler, W. Therapeutic potential and limitations of new FAK inhibitors in the treatment of cancer. Expert Opin. Investig. Drugs 19, 777–788 (2010).
Lagares, D. et al. Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum. 64, 1653–1664 (2012).
Akhmetshina, A. et al. Rho-associated kinases are crucial for myofibroblast differentiation and production of extracellular matrix in scleroderma fibroblasts. Arthritis Rheum. 58, 2553–2564 (2008).
Huang, X. et al. Relaxin regulates myofibroblast contractility and protects against lung fibrosis. Am. J. Pathol. 179, 2751–2765 (2011).
Jiang, C. et al. Fasudil, a rho-kinase inhibitor, attenuates bleomycin-induced pulmonary fibrosis in mice. Int. J. Mol. Sci. 13, 8293–8307 (2012).
Washida, N. et al. Rho-kinase inhibition ameliorates peritoneal fibrosis and angiogenesis in a rat model of peritoneal sclerosis. Nephrol. Dial. Transplant. 26, 2770–2779 (2011).
Ishimaru, K. et al. Fasudil attenuates myocardial fibrosis in association with inhibition of monocyte/macrophage infiltration in the heart of DOCA/salt hypertensive rats. J. Cardiovasc. Pharmacol. 50, 187–194 (2007).
Zhou, Y. et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J. Clin. Investig. 123, 1096–1108 (2013).
Velasquez, L. S. et al. Activation of MRTF-A-dependent gene expression with a small molecule promotes myofibroblast differentiation and wound healing. Proc. Natl Acad. Sci. USA 110, 16850–16855 (2013).
Sakai, N. et al. LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J. 27, 1830–1846 (2013).
Small, E. M. et al. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circulation Res. 107, 294–304 (2010).
Luchsinger, L. L., Patenaude, C. A., Smith, B. D. & Layne, M. D. Myocardin-related transcription factor-A complexes activate type I collagen expression in lung fibroblasts. J. Biol. Chem. 286, 44116–44125 (2011).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Yu, F. X. et al. Regulation of the Hippo-YAP pathway by G.-protein-coupled receptor signaling. Cell 150, 780–791 (2012).
Varelas, X. et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat. Cell Biol. 10, 837–848 (2008).
Liu-Chittenden, Y. et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26, 1300–1305 (2012).
Denton, C. P. & Ong, V. H. Targeted therapies for systemic sclerosis. Nat. Rev. Rheumatol. 9, 451–464 (2013).
Acknowledgements
M.K. gratefully acknowledges support from the Canadian Institute of Health Research Operating Grants. D.L. and A.M.T. gratefully acknowledge support from NIH Heart Lung and Blood Institute–NIH grants R01-HL095732 and R01-HL108975, and from the Scleroderma Research Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching data for the article, providing a substantial contribution to discussions of the content, writing the article, and to the review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ho, Y., Lagares, D., Tager, A. et al. Fibrosis—a lethal component of systemic sclerosis. Nat Rev Rheumatol 10, 390–402 (2014). https://doi.org/10.1038/nrrheum.2014.53
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.53
This article is cited by
-
Mesenchymal stem cell-based therapy for autoimmune-related fibrotic skin diseases—systemic sclerosis and sclerodermatous graft-versus-host disease
Stem Cell Research & Therapy (2023)
-
Heavy-chain antibody targeting of CD38 NAD+ hydrolase ectoenzyme to prevent fibrosis in multiple organs
Scientific Reports (2023)
-
Increased rate of complications following total knee arthroplasty in patients with systemic sclerosis
International Orthopaedics (2023)
-
FOXO4-D-Retro-Inverso targets extracellular matrix production in fibroblasts and ameliorates bleomycin-induced pulmonary fibrosis in mice
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Soluble guanylate cyclase stimulator reduced the gastrointestinal fibrosis in bleomycin-induced mouse model of systemic sclerosis
Arthritis Research & Therapy (2021)