Abstract
In this Review we describe three approaches for cartilage tissue repair at the rheumatology–orthopaedics interface: disease-modifying osteoarthritis (OA) drug (DMOAD) treatment; cell-based therapies, and intrinsic cartilage repair by joint distraction. DMOADs can slow the progression of joint damage. Cell-based therapies have evolved to do the same, through selection of the most potent cell types (and combinations thereof), as well as identification of permissive boundary conditions for indications. Joint distraction techniques, meanwhile, have now demonstrated the capacity to stimulate actual intrinsic tissue repair. Although this progress is promising, true biological joint reconstruction remains distant on the developmental pathway of 'regenerative medicine'. Prolonged functional repair—that is, cure of diseases such as OA—remains an unmet medical need and scientific challenge, for which comparative and constructive interaction between these physical, chemical and cellular approaches will be required. Careful selections of patients and combinations of approaches will need to be made and tested to demonstrate their cost-effectiveness. Only with such rational and integrated assessment of outcomes will the promising results of these approaches be consolidated in clinical practice.
Key Points
-
The quest for disease-modifying osteoarthritis drugs (DMOADs) is becoming increasingly fruitful; modalities that alter bone turnover—and, indirectly, cartilage damage—seem to be most effective
-
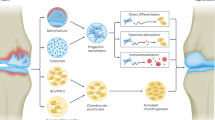
Long-term outcomes of cell-based therapies are good; quality has improved with European advanced therapeutic medicinal products regulation; the current goal is combining cartilage components, mesenchymal stem cells and trophic factors into a one-stage therapy
-
Joint distraction can induce tissue-structure modification in degenerated knee joints, accompanied by prolonged symptomatic improvement that supports the concept of cartilage repair translating into real clinical benefit
-
Joint distraction itself might represent an integrated approach to tackling the separate chondroprotective, chondroreparative and bone turnover-modifying mechanisms targeted by DMOADs and cell-based therapies
-
Combining DMOAD and cell-based therapies with joint distraction might be a worthwhile approach towards functional tissue repair, as distraction provides a temporary biomechanical joint homeostasis that facilities repair mechanisms
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Report No. CPMP/EWP/784/97 (Committee for Proprietary Medicinal Products [CPMP], London, 1998).
FDA. Guidance for induistry: clinical development programs for drugs, devices and biological products intended for the treatment of OA. U.S. Food and Drug Administration [online], (1999).
Mastbergen, S. C. & Lafeber, F. P. Animal models of osteoarthritis—why choose a larger model? US Musculoskeletal Review 4, 11–14 (2009).
Bingham, C. O. 3rd. et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 54, 3494–3507 (2006).
Bruyere, O. et al. Total joint replacement after glucosamine sulphate treatment in knee osteoarthritis: results of a mean 8-year observation of patients from two previous 3-year, randomised, placebo-controlled trials. Osteoarthritis Cartilage 16, 254–260 (2008).
Dougados, M. et al. Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis: ECHODIAH, a three-year, placebo-controlled trial. Evaluation of the Chondromodulating Effect of Diacerein in OA of the Hip. Arthritis Rheum. 44, 2539–2547 (2001).
Manno, R. L. et al. OARSI-OMERACT initiative: defining thresholds for symptomatic severity and structural changes in disease modifying osteoarthritis drug (DMOAD) clinical trials. Osteoarthritis 20, 93–101 (2012).
Wluka, A. E., Wolfe, R., Stuckey, S. & Cicuttini, F. M. How does tibial cartilage volume relate to symptoms in subjects with knee osteoarthritis? Ann. Rheum. Dis. 63, 264–268 (2004).
Cicuttini, F. M., Jones, G., Forbes, A. & Wluka, A. E. Rate of cartilage loss at two years predicts subsequent total knee arthroplasty: a prospective study. Ann. Rheum. Dis. 63, 1124–1127 (2004).
Bauer, D. C. et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage 14, 723–727 (2006).
Hunter, D. J. Pharmacologic therapy for osteoarthritis--the era of disease modification. Nat. Rev. Rheumatol. 7, 13–22 (2011).
Martel-Pelletier, J., Wildi, L. M. & Pelletier, J. P. Future therapeutics for osteoarthritis. Bone 51, 297–311 (2012).
Hellio le Graverand, M. P. et al. A 2-year randomized, double-blind, placebo controlled, multicenter study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann. Rheum. Dis. 72, 187–195 (2012).
Pelletier, J. P. et al. Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis Rheum. 43, 1290–1299 (2000).
Bijlsma, J. W., Berenbaum, F. & Lafeber, F. P. Osteoarthritis: an update with relevance for clinical practice. Lancet 377, 2115–2126 (2011).
Baragi, V. M. et al. A new class of potent matrix metalloproteinase 13 inhibitors for potential treatment of osteoarthritis: Evidence of histologic and clinical efficacy without musculoskeletal toxicity in rat models. Arthritis Rheum. 60, 2008–2018 (2009).
Fosang, A. Knock-in mice reveal in vivo consequences of MMP activity for OA [abstract SP0022]. Ann. Rheum. Dis. 71 (Suppl. 3), 6 (2012).
Hunter, D. J. et al. Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC Musculoskelet. Disord. 11, 232 (2010).
Haque, T., Nakada, S. & Hamdy, R. C. A review of FGF18: Its expression, signaling pathways and possible functions during embryogenesis and post-natal development. Histol. Histopathol. 22, 97–105 (2007).
Ellman, M. B., An, H. S., Muddasani, P. & Im, H. J. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene 420, 82–89 (2008).
Moore, E. E. et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage 13, 623–631 (2005).
McPherson, R., Flechsenhar, K., Hellot, S. & Eckstein, F. A randomized, double blind, placebo-controlled, multicenter study of rhFGF18 administered intraarticularly using single or multiple ascending doses in patients with primary knee osteoarthritis (OA), not expected to require knee surgery within 1 year. Osteoarthritis Cartilage 19 (Suppl. 1), 35–36 (2011).
Karsdal, M. A., Sondergaard, B. C., Arnold, M. & Christiansen, C. Calcitonin affects both bone and cartilage: a dual action treatment for osteoarthritis? Ann. N. Y. Acad. Sci. 1117, 181–195 (2007).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Karsdal, M. A. et al. Oral calcitonin demonstrated symptom-modifying efficacy and increased cartilage volume: results from a 2-year phase 3 trial in patients with osteoarthritis of the knee. Osteoarthritis Cartilage 19 (Suppl. 1), 35 (2011).
Marie, P. J., Ammann, P., Boivin, G. & Rey, C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif. Tissue Int. 69, 121–129 (2001).
Henrotin, Y. et al. Strontium ranelate increases cartilage matrix formation. J. Bone Miner. Res. 16, 299–308 (2001).
Alexandersen, P., Karsdal, M. A., Byrjalsen, I. & Christiansen, C. Strontium ranelate effect in postmenopausal women with different clinical levels of osteoarthritis. Climacteric 14, 236–243 (2011).
Cooper, C. et al. Efficacy and safety of oral strontium ranelate for the treatment of knee osteoarthritis: rationale and design of randomised, double-blind, placebo-controlled trial. Curr. Med. Res. Opin. 28, 231–239 (2012).
Reginster, J. Y. et al. Efficacy and saftey of strontium ranelate in the treatment of knee osteoarthritis: results of a double-blind, randomised placebo-controlled trial. Ann. Rheum. Dis. 72, 179–186 (2013).
Pelletier, J. P. et al. Strontium ranelate reduces the progression of experimental dog osteoarthritis by inhibiting the expression of key proteases in cartilage and of IL-1beta in the synovium. Ann. Rheum. Dis. 72, 250–257 (2013).
Li, Y. et al. Effects of strontium on proliferation and differentiation of rat bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 418, 725–730 (2012).
Lafeber F. P. & van Laar J. M. Strontium ranelate: ready for clinical use as disease-modifying osteoarthritis drug? Ann. Rheum. Dis. 72, 157–161 (2013).
Guermazi, A., Roemer, F. W. & Hayashi, D. Imaging of osteoarthritis: update from a radiological perspective. Curr. Opin. Rheumatol. 23, 484–491 (2011).
van Spil, W. E., DeGroot, J., Lems, W. F., Oostveen, J. C. & Lafeber, F. P. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthritis Cartilage 18, 605–612 (2010).
Buckwalter, J. A. & Mankin, H. J. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr. Course Lect. 47, 487–504 (1998).
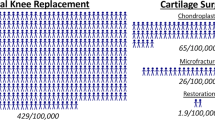
Harris, J. D., Siston, R. A., Pan, X. & Flanigan, D. C. Autologous chondrocyte implantation: a systematic review. J. Bone Joint Surg. Am. 92, 2220–2233 (2010).
Lindahl, A., Brittberg, M. & Peterson, L. Health economics benefits following autologous chondrocyte transplantation for patients with focal chondral lesions of the knee. Knee Surg. Sports Traumatol. Arthrosc. 9, 358–363 (2001).
Brittberg, M. ... More bricks to the building of cartilage knowledge? Knee Surg. Sports Traumatol. Arthrosc. 17, 1275–1277 (2009).
Brittberg, M. et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 331, 889–895 (1994).
Bentley, G. et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J. Bone Joint Surg. Br. 85, 223–230 (2003).
Jakobsen, R. B., Engebretsen, L. & Slauterbeck, J. R. An analysis of the quality of cartilage repair studies. J. Bone Joint Surg. Am. 87, 2232–2239 (2005).
Lohmander, L. S. Articular cartilage and osteoarthrosis. The role of molecular markers to monitor breakdown, repair and disease. J. Anat. 184 (Pt 3), 477–492 (1994).
Saris, D. B., Dhert, W. J. & Verbout, A. J. Joint homeostasis. The discrepancy between old and fresh defects in cartilage repair. J. Bone Joint Surg. Br. 85, 1067–1076 (2003).
Marijnissen, A. C. & Lafeber, F. P. Re: E. B. Hunziker. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis and Cartilage 2002; 10, 432–463 Osteoarthritis Cartilage 11, 300–301; author reply 302–304 (2003).
Zeifang, F. et al. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am. J. Sports Med. 38, 924–933 (2010).
de Windt, T. S., Concaro, S., Lindahl, A., Saris, D. B. & Brittberg, M. Strategies for patient profiling in articular cartilage repair of the knee: a prospective cohort of patients treated by one experienced cartilage surgeon. Knee Surg. Sports Traumatol. Arthrosc. 20, 2225–2232 (2012).
Filardo, G., Kon, E., Di Martino, A., Iacono, F. & Marcacci, M. Arthroscopic second-generation autologous chondrocyte implantation: a prospective 7-year follow-up study. Am. J. Sports Med. 39, 2153–2160 (2011).
Kon, E. et al. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am. J. Sports Med. 37, 33–41 (2009).
Saris, D. B. et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am. J. Sports Med. 36, 235–246 (2008).
Roos, E. M. & Lohmander, L. S. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual. Life Outcomes 1, 64 (2003).
Roos, E. M., Roos, H. P., Lohmander, L. S., Ekdahl, C. & Beynnon, B. D. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J. Orthop. Sports Phys. Ther. 28, 88–96 (1998).
Knutsen, G. et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J. Bone Joint Surg. Am. 89, 2105–2112 (2007).
Knutsen, G. et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J. Bone Joint Surg. Am. 86-A, 455–464 (2004).
Peterson, L. et al. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin. Orthop. Relat. Res. 212–234 (2000).
Saris, D. B. et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am. J. Sports Med. 37 (Suppl. 1), 10–19 (2009).
Vanlauwe, J. et al. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am. J. Sports Med. 39, 2566–2574 (2011).
Bekkers, J. E., Inklaar, M. & Saris, D. B. Treatment selection in articular cartilage lesions of the knee: a systematic review. Am. J. Sports Med. 37 (Suppl. 1), 148–55 (2009).
de Windt, T. S., Bekkers, J. E., Creemers, L. B., Dhert, W. J. & Saris, D. B. Patient profiling in cartilage regeneration: prognostic factors determining success of treatment for cartilage defects. Am. J. Sports Med. 37 (Suppl. 1), 58–62 (2009).
Hendriks, J., Riesle, J. & van Blitterswijk, C. A. Co-culture in cartilage tissue engineering. J. Tissue Eng. Regen. Med. 1, 170–178 (2007).
Leijten, J. C., Georgi, N., Wu, L., van Blitterswijk, C. A. & Karperien, M. Cell sources for articular cartilage repair strategies: shifting from monocultures to cocultures. Tissue Eng. Part B Rev. 19, 31–49 (2013).
Mo, X. T. et al. Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone 45, 42–51 (2009).
Wu, L. et al. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng. Part A 17, 1425–1436 (2011).
European Medicines Agency. EU Clinical Trials Register [online], (2012).
Lee, C. H. et al. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet 376, 440–448 (2010).
Sekiya, I. et al. Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. J. Orthop. Res. 30, 943–949 (2012).
Gudas, R. et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am. J. Sports Med. 4, 2499–2508 (2012).
Centeno, C. J. et al. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr. Stem Cell Res. Ther. 5, 81–93 (2010).
Davatchi, F., Abdollahi, B. S., Mohyeddin, M., Shahram, F. & Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 14, 211–215 (2011).
Koh, Y. G. & Choi, Y. J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 19, 902–907 (2012).
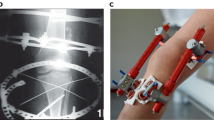
Lafeber, F. P., Intema, F., Van Roermund, P. M. & Marijnissen, A. C. Unloading joints to treat osteoarthritis, including joint distraction. Curr. Opin. Rheumatol. 18, 519–525 (2006).
Intema, F. et al. Subchondral bone remodeling is related to clinical improvement after joint distraction in the treatment of ankle osteoarthritis. Osteoarthritis Cartilage 19, 668–675 (2011).
Kanbe, K., Hasegawa, A., Takagishi, K., Kaneko, H. & Nakajima, Y. Arthroscopic findings of the joint distraction for the patient with chondrolysis of the ankle. Diagn. Ther. Endosc. 4, 101–105 (1997).
Lamm, B. M. & Gourdine-Shaw, M. MRI evaluation of ankle distraction: a preliminary report. Clin. Podiatr. Med. Surg. 26, 185–191 (2009).
Marijnissen, A. C. et al. Clinical benefit of joint distraction in the treatment of severe osteoarthritis of the ankle: proof of concept in an open prospective study and in a randomized controlled study. Arthritis Rheum. 46, 2893–2902 (2002).
Paley, D., Lamm, B. M., Purohit, R. M. & Specht, S. C. Distraction arthroplasty of the ankle--how far can you stretch the indications? Foot Ankle Clin. 13, 471–484, ix (2008).
Ploegmakers, J. J. et al. Prolonged clinical benefit from joint distraction in the treatment of ankle osteoarthritis. Osteoarthritis Cartilage 13, 582–588 (2005).
Sabharwal, S. & Schwechter, E. M. Five-year followup of ankle joint distraction for post-traumatic chondrolysis in an adolescent: a case report. Foot Ankle Int. 28, 942–948 (2007).
Saltzman, C. L., Hillis, S. L., Stolley, M. P., Anderson, D. D. & Amendola, A. Motion versus fixed distraction of the joint in the treatment of ankle osteoarthritis: a prospective randomized controlled trial. J. Bone Joint Surg. Am. 94, 961–970 (2012).
Tellisi, N., Fragomen, A. T., Kleinman, D., O'Malley, M. J. & Rozbruch, S. R. Joint preservation of the osteoarthritic ankle using distraction arthroplasty. Foot Ankle Int. 30, 318–325 (2009).
Van Meegeren, M. E. et al. Joint distraction results in clinical and structural improvement of haemophilic ankle arthropathy: a series of three cases. Haemophilia 18, 810–817 (2012).
van Valburg, A. A. et al. Can Ilizarov joint distraction delay the need for an arthrodesis of the ankle? A preliminary report. J. Bone Joint Surg. Br. 77, 720–725 (1995).
van Valburg, A. A. et al. Joint distraction in treatment of osteoarthritis: a two-year follow-up of the ankle. Osteoarthritis Cartilage. 7, 474–479 (1999).
Abouheif, M. M. et al. Repair of a large osteochondral defect in the knee joint using autologous and artificial bone graft combined with motion preserving distraction arthroplasty: a case report. Arch. Orthop. Trauma Surg. 130, 231–236 (2010).
Aldegheri, R., Trivella, G. & Saleh, M. Articulated distraction of the hip. Conservative surgery for arthritis in young patients. Clin. Orthop. Relat. Res. 94–101 (1994).
Aly, T. A., Hafez, K. & Amin, O. Arthrodiatasis for management of knee osteoarthritis. Orthopedics 34, e338–e343 (2011).
Deie, M. et al. Knee articulated distraction arthroplasty for the middle-aged osteoarthritic knee joint. Tech. Knee Surg. 9, 80–84 (2010).
Deie, M., Ochi, M., Adachi, N., Kajiwara, R. & Kanaya, A. A new articulated distraction arthroplasty device for treatment of the osteoarthritic knee joint: a preliminary report. Arthroscopy 23, 833–838 (2007).
Gomez, J. A. et al. Articulated hip distraction: a treatment option for femoral head avascular necrosis in adolescence. J. Pediatr. Orthop. 29, 163–169 (2009).
Intema, F. et al. Tissue structure modification in knee osteoarthritis by use of joint distraction: an open 1-year pilot study. Ann. Rheum. Dis. 70, 1441–1446 (2011).
Thacker, M. M., Feldman, D. S., Madan, S. S., Straight, J. J. & Scher, D. M. Hinged distraction of the adolescent arthritic hip. J. Pediatr. Orthop. 25, 178–182 (2005).
Koshino, T., Wada, S., Ara, Y. & Saito, T. Regeneration of degenerated articular cartilage after high tibial valgus osteotomy for medial compartmental osteoarthritis of the knee. Knee 10, 229–236 (2003).
Marijnissen, A. C. et al. Knee Images Digital Analysis (KIDA): a novel method to quantify individual radiographic features of knee osteoarthritis in detail. Osteoarthritis Cartilage 16, 234–243 (2008).
Wiegant, K. et al. Structural tissue changes and prolonged clinical improvement by joint distraction in treatment of end-stage knee osteoarthritis; the 2 years follow-up. Osteoarthritis Cartilage, 19 (Suppl. 1), 36 (2011).
Bain, G. I., Mehta, J. A., Heptinstall, R. J. & Bria, M. Dynamic external fixation for injuries of the proximal interphalangeal joint. J. Bone Joint Ssurg. Br. 80, 1014–1019 (1998).
DeVries, J. G., Amiot, R. A., Cummings, P. & Sockrider, N. Freiberg's infraction of the second metatarsal treated with autologous osteochondral transplantation and external fixation. J. Foot Ankle Surg. 47, 565–570 (2008).
van Roermund, P. M. et al. Function of stiff joints may be restored by Ilizarov joint distraction. Clin. Orthop. Relat. Res. 220–227 (1998).
Judet, R. & Judet, T. [The use of a hinge distraction apparatus after arthrolysis and arthroplasty (author's transl)]. Revue de chirurgie orthopedique et reparatrice de l'appareil moteur [French] 64, 353–365 (1978).
Cañadell, J., Gonzales, F., Barrios, R. H. & Amillo, S. Arthrodiastasis for stiff hips in young patients. Int. Orthop. 17, 254–258 (1993).
Morrey, B. F. Posttraumatic stiffness: distraction arthroplasty. Orthopedics 15, 863–869 (1992).
Clifford, A., O'Connell, M., Gabriel, S., Miller, L. E. & Block, J. E. The KineSpring load absorber implant: rationale, design and biomechanical characterization. J. Med. Eng. Technol. 35, 65–71 (2011).
Allen, M. J. et al. Evaluation of the safety of a novel knee load-bypassing device in a sheep model. J. Bone Joint Surg. Am. 94, 77–84 (2012).
Kajiwara, R. et al. Effective repair of a fresh osteochondral defect in the rabbit knee joint by articulated joint distraction following subchondral drilling. J. Orthop. Res. 23, 909–915 (2005).
Nishino, T. et al. Joint distraction and movement for repair of articular cartilage in a rabbit model with subsequent weight-bearing. J. Bone Joint Surg. Br. 92, 1033–1040 (2010).
Nishino, T. et al. Effect of gradual weight-bearing on regenerated articular cartilage after joint distraction and motion in a rabbit model. J. Orthop. Res. 28, 600–606 (2010).
Yanai, T., Ishii, T., Chang, F. & Ochiai, N. Repair of large full-thickness articular cartilage defects in the rabbit: the effects of joint distraction and autologous bone-marrow-derived mesenchymal cell transplantation. J. Bone Joint Surg. Br. 87, 721–729 (2005).
van Valburg, A. A. et al. Joint distraction in treatment of osteoarthritis (II): effects on cartilage in a canine model. Osteoarthritis Cartilage 8, 1–8 (2000).
Karadam, B., Karatosun, V., Murat, N., Ozkal, S. & Gunal, I. No beneficial effects of joint distraction on early microscopical changes in osteoarthrotic knees. A study in rabbits. Acta Orthop. 76, 95–98 (2005).
Kroeber, M. et al. Effects of controlled dynamic disc distraction on degenerated intervertebral discs: an in vivo study on the rabbit lumbar spine model. Spine (Phila Pa 1976) 30, 181–187 (2005).
McAlindon, T. E., Driban, J. B. & Lo, G. H. Osteoarthritis year 2011 in review: clinical. Osteoarthritis Cartilage 20, 197–200 (2012).
Parker, D. A., Beatty, K. T., Giuffre, B., Scholes, C. J. & Coolican, M. R. Articular cartilage changes in patients with osteoarthritis after osteotomy. Am. J. Sports Med. 39, 1039–1045 (2011).
Burr, D. B. & Gallant, M. A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 8, 665–673 (2012).
Jones, E. A. et al. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: detection and functional evaluation at the single-cell level. Arthritis Rheum. 58, 1731–1740 (2008).
Jones, E. A. et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 46, 3349–3360 (2002).
Koelling, S. et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 4, 324–335 (2009).
Vinardell, T., Buckley, C. T., Thorpe, S. D. & Kelly, D. J. Composition-function relations of cartilaginous tissues engineered from chondrocytes and mesenchymal stem cells isolated from bone marrow and infrapatellar fat pad. J. Tissue Eng. Regen. Med. 5, 673–683 (2011).
Richter, W. Mesenchymal stem cells and cartilage in situ regeneration. J. Intern. Med. 266, 390–405 (2009).
Grad, S., Eglin, D., Alini, M. & Stoddart, M. J. Physical stimulation of chondrogenic cells in vitro: a review. Clin. Orthop. Relat Res. 469, 2764–2772 (2011).
Nohmi, S. et al. Post injury changes in the properties of mesenchymal stem cells derived from human anterior cruciate ligaments. Int. Orthop. 36, 1515–1522 (2012).
Mobasheri, A., Csaki, C., Clutterbuck, A. L., Rahmanzadeh, M. & Shakibaei, M. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: applications in cartilage repair and osteoarthritis therapy. Histol. Histopathol. 24, 347–366 (2009).
Spector, T. D. et al. Effect of risedronate on joint structure and symptoms of knee osteoarthritis: results of the BRISK randomized, controlled trial [ISRCTN01928173]. Arthritis Res. Ther. 7, R625–R633 (2005).
Brandt, K. D., Mazzuca, S. A., Katz, B. P. et al. Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 52, 2015–2025 (2005).
Pavelká, K. et al. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch. Intern. Med. 162, 2113–2123 (2002).
Kahan, A., Uebelhart, D., De Vathaire, F., Delmas, P. D. & Reginster, J. Y. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 60, 524–533 (2009).
Sawitzke, A. D. et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheum. 58, 3183–3191 (2008).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
Krzeski, P. et al. Development of musculoskeletal toxicity without clear benefit after administration of PG-116800, a matrix metalloproteinase inhibitor, to patients with knee osteoarthritis: a randomized, 12-month, double-blind, placebo-controlled study. Arthritis Res. Ther. 9, R109 (2007).
US National Library of Medicine. ClinicalTrials.gov [online], (2007).
Acknowledgements
We thank A. M. van Laar for the literature search.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to researching data for the article, writing the article and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D. B. F. Saris declares that he has acted as a consultant for, received speakers' honoraria from, and received grant/research support from Sanofi and Smith & Nephew, has acted as a consultant for Regentis, and has a teaching agreement with and has received grant support from TiGenix. S. C. Mastbergen and F. P. J. G. Lafeber declare no competing interests.
Supplementary information
Supplementary Table 1
Studies of joint distraction in animal models of knee joint and vertebral disc degeneration. (DOC 55 kb)
Supplementary Figure 1
Search, inclusion and exclusion strategies applied to the identification of publications relevant to the section on joint distraction. (DOC 330 kb)
Rights and permissions
About this article
Cite this article
Mastbergen, S., Saris, D. & Lafeber, F. Functional articular cartilage repair: here, near, or is the best approach not yet clear?. Nat Rev Rheumatol 9, 277–290 (2013). https://doi.org/10.1038/nrrheum.2013.29
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2013.29
This article is cited by
-
Mechanical osteoarthritis of the hip in a one medicine concept: a narrative review
BMC Veterinary Research (2023)
-
Failure of cartilage regeneration: emerging hypotheses and related therapeutic strategies
Nature Reviews Rheumatology (2023)
-
Suitable characteristics in the selection of human allogeneic chondrocytes donors to increase the number of viable cells for cartilage repair
Cell and Tissue Banking (2023)
-
Mesenchymal stem cells augmentation for surgical procedures in patients with symptomatic chondral defects of the knee: a systematic review
Journal of Orthopaedic Surgery and Research (2022)
-
Joint distraction for osteoarthritis: clinical evidence and molecular mechanisms
Nature Reviews Rheumatology (2022)