Abstract
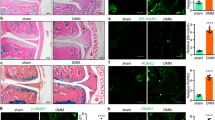
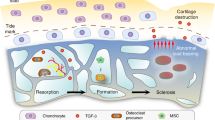
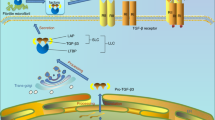
During osteoarthritis (OA), angiogenesis is increased in the synovium, osteophytes and menisci and leads to ossification in osteophytes and the deep layers of articular cartilage. Angiogenic and antiangiogenic factors might both be upregulated in the osteoarthritic joint; however, vascular growth predominates, and the articular cartilage loses its resistance to vascularization. In addition, blood vessel growth is increased at—and disrupts—the osteochondral junction. Angiogenesis in this location is dependent on the creation of channels from subchondral bone spaces into noncalcified articular cartilage. Inflammation drives synovial angiogenesis through macrophage activation. Blood vessel and nerve growth are linked by common pathways that involve the release of proangiogenic factors, such as vascular endothelial growth factor, β-nerve growth factor and neuropeptides. Proangiogenic factors might also stimulate nerve growth, and molecules produced by vascular cells could both stimulate and guide nerve growth. As sensory nerves grow along new blood vessels in osteoarthritic joints, they eventually penetrate noncalcified articular cartilage, osteophytes and the inner regions of menisci. Angiogenesis could, therefore, contribute to structural damage and pain in OA and provide potential targets for new treatments.
Key Points
-
Angiogenesis contributes to synovitis, osteochondral damage, osteophyte formation and meniscal pathology in patients with osteoarthritis (OA)
-
Angiogenesis is intimately linked to sensory nerve growth through shared regulatory pathways
-
Nerve growth along new blood vessels into structures that are normally not innervated could contribute to pain in OA
-
Inhibition of subchondral bone turnover can prevent osteochondral angiogenesis and reduce pain in animal models of OA
-
Inhibition of angiogenesis could potentially be used to treat OA
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brown, R. A. & Weiss, J. B. Neovascularisation and its role in the osteoarthritic process. Ann. Rheum. Dis. 47, 881–885 (1988).
Fransès, R. E., McWilliams, D. F., Mapp., P. I. & Walsh, D. A. Osteochondral angiogenesis and increased protease inhibitor expression in OA. Osteoarthritis Cartilage 18, 563–571 (2010).
Bonnet, C. S. & Walsh, D. A. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 44, 7–16 (2005).
Ashraf, S. et al. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann. Rheum. Dis. 70, 523–529 (2011).
Levick, J. R. & Knight, A. D. Osmotic flows across the blood-joint barrier. Ann. Rheum. Dis. 46, 534–539 (1987).
Stevens, C. R., Blake, D. R., Merry, P., Revell, P. A. & Levick, J. R. A comparative study by morphometry of the microvasculature in normal and rheumatoid synovium. Arthritis Rheum. 34, 1508–1513 (1991).
Blake, D. R. et al. Hypoxic-reperfusion injury in the inflamed human joint. Lancet 1, 289–293 (1989).
O'Hara, B. P., Urban, J. P. & Maroudas, A. Influence of cyclic loading on the nutrition of articular cartilage. Ann. Rheum. Dis. 49, 536–539 (1990).
Walsh, D. A., Mapp., P. I., Wharton, J., Polak, J. M. & Blake, D. R. Neuropeptide degrading enzymes in normal and inflamed human synovium. Am. J. Pathol. 142, 1610–1621 (1993).
Suri, S. et al. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann. Rheum. Dis. 66, 1423–1428 (2007).
Ashraf, S., Mapp., P. I. & Walsh, D. A. Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis Rheum. 63, 2700–2710 (2011).
Mapp., P. I. et al. Angiogenesis in two animal models of osteoarthritis. Osteoarthritis Cartilage 16, 61–69 (2008).
Shibakawa, A. et al. Presence of pannus-like tissue on osteoarthritic cartilage and its histological character. Osteoarthritis Cartilage 11, 133–140 (2003).
Smith, M. D., Triantafillou, S., Parker, A., Youssef, P. P. & Coleman, M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J. Rheumatol. 24, 365–371 (1997).
Bondeson, J. et al. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 62, 647–657 (2010).
Haywood, L. et al. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 48, 2173–2177 (2003).
Conaghan, P. G. et al. MRI and non-cartilaginous structures in knee osteoarthritis. Osteoarthritis Cartilage 14 (Suppl. A), A87–A94 (2006).
Revell, P. A., Mayston, V., Lalor, P. & Mapp., P. The synovial membrane in osteoarthritis: a histological study including the characterisation of the cellular infiltrate present in inflammatory osteoarthritis using monoclonal antibodies. Ann. Rheum. Dis. 47, 300–307 (1988).
Walsh, D. A., Wade, M., Mapp., P. I. & Blake, D. R. Focally regulated endothelial proliferation and cell death in human synovium. Am. J. Pathol. 152, 691–702 (1998).
Walsh, D. A. et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford) 49, 1852–1861 (2010).
Petersen, W. & Tillmann, B. Collagenous fibril texture of the human knee joint menisci. Anat. Embryol. (Berl.) 197, 317–324 (1998).
Aigner, T., Dietz, U., Stöss, H. & von der Mark, K. Differential expression of collagen types I, II, III, and X in human osteophytes. Lab. Invest. 73, 236–243 (1995).
Hashimoto, S. et al. Development and regulation of osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage 10, 180–187 (2002).
Lainer-Carr, D. & Brahn, E. Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis. Nat. Clin. Pract. Rheumatol. 3, 434–442 (2007).
Walsh, D. A. et al. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis Cartilage 15, 743–751 (2007).
Mantovani, A., Sica, A. & Locati, M. Macrophage polarization comes of age. Immunity 23, 344–346 (2005).
Aron-Wisnewsky, J. et al. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J. Clin. Endocrinol. Metab. 94, 4619–4623 (2009).
Hamilton, J. A. & Tak, P. P. The dynamics of macrophage lineage populations in inflammatory and autoimmune diseases. Arthritis Rheum. 60, 1210–1221 (2009).
McInnes, I. B. et al. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J. Exp. Med. 184, 1519–1524 (1996).
Wu, W. K., Llewellyn, O. P., Bates, D. O., Nicholson, L. B. & Dick, A. D. IL-10 regulation of macrophage VEGF production is dependent on macrophage polarisation and hypoxia. Immunobiology 215, 796–803 (2010).
Pufe, T., Petersen, W., Tillmann, B. & Mentlein, R. The splice variants VEGF121 and VEGF189 of the angiogenic peptide vascular endothelial growth factor are expressed in osteoarthritic cartilage. Arthritis Rheum. 44, 1082–1088 (2001).
Pfander, D., Cramer, T., Deuerling, D., Weseloh, G. & Swoboda, B. Expression of thrombospondin-1 and its receptor CD36 in human osteoarthritic cartilage. Ann. Rheum. Dis. 59, 448–454 (2000).
Moses, M. A. et al. Troponin I is present in human cartilage and inhibits angiogenesis. Proc. Natl Acad. Sci. USA 96, 2645–2650 (1999).
Eisenstein, R., Sorgente, N., Soble, L. W., Miller, A. & Kuettner, K. E. The resistance of certain tissues to invasion: penetrability of explanted tissues by vascularized mesenchyme. Am. J. Pathol. 73, 765–774 (1973).
Fenwick, S. A., Gregg, P. J. & Rooney, P. Osteoarthritic cartilage loses its ability to remain avascular. Osteoarthritis Cartilage 7, 441–452 (1999).
Walker, G. D., Fischer, M., Gannon, J., Thompson, R. C. Jr & Oegema, T. R. Jr. Expression of type-X collagen in osteoarthritis. J. Orthop. Res. 13, 4–12 (1995).
Wilson, A. S., Legg, P. G. & McNeur, J. C. Studies on the innervation of the medial meniscus in the human knee joint. Anat. Rec. 165, 485–491 (1969).
Day, B., Mackenzie, W. G., Shim, S. S. & Leung, G. The vascular and nerve supply of the human meniscus. Arthroscopy 1, 58–62 (1985).
Mapp., P. I. Innervation of the synovium. Ann. Rheum. Dis. 54, 398–403 (1995).
Lanzetta, A. et al. The nervous structures of anterior cruciate ligament of human knee, healthy and lesioned, studied with confocal scanning laser microscopy. Ital. J. Anat. Embryol. 109, 167–176 (2004).
Reimann, I. & Christensen, S. B. A histological demonstration of nerves in subchondral bone. Acta Orthop. Scand. 48, 345–352 (1977).
Mapp., P. I. et al. Substance P-, calcitonin gene-related peptide- and C-flanking peptide of neuropeptide Y-immunoreactive fibres are present in normal synovium but depleted in patients with rheumatoid arthritis. Neuroscience 37, 143–153 (1990).
Walsh, D. A. et al. Innervation and neurokinin receptors during angiogenesis in the rat sponge granuloma. Histochem. J. 28, 759–769 (1996).
Jimenez-Andrade, J. M., Ghilardi, J. R., Castañeda-Corral, G., Kuskowski, M. A. & Mantyh, P. W. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 152, 2564–2574 (2011).
Fan, T. P., Hu, D. E., Guard, S., Gresham, G. A. & Watling, K. J. Stimulation of angiogenesis by substance P and interleukin-1 in the rat and its inhibition by NK1 or interleukin-1 receptor antagonists. Br. J. Pharmacol. 110, 43–49 (1993).
Movafagh, S., Hobson, J. P., Spiegel, S., Kleinman, H. K. & Zukowska, Z. Neuropeptide Y induces migration, proliferation, and tube formation of endothelial cells bimodally via Y1, Y2, and Y5 receptors. FASEB J. 20, 1924–1926 (2006).
Seegers, H. C., Hood, V. C., Kidd, B. L., Cruwys, S. C. & Walsh, D. A. Enhancement of angiogenesis by endogenous substance P release and neurokinin-1 receptors during neurogenic inflammation. J. Pharmacol. Exp. Ther. 306, 8–12 (2003).
Seegers, H. C., Avery, P. S., McWilliams, D. F., Haywood, L. & Walsh, D. A. Combined effect of bradykinin B2 and neurokinin-1 receptor activation on endothelial cell proliferation in acute synovitis. FASEB J. 18, 762–764 (2004).
Mapp., P. I., McWilliams, D. F., Turley, M. J., Hargin, E. & Walsh, D. A. A role for the sensory neuropeptide calcitonin gene-related peptide in endothelial cell proliferation in vivo. Br. J. Pharmacol. 166, 1261–1271 (2012).
Ashraf, S., Mapp., P. I. & Walsh, D. A. Angiogenesis and the persistence of inflammation in a rat model of proliferative synovitis. Arthritis Rheum. 62, 1890–1898 (2010).
Carmeliet, P. & Tessier-Lavigne, M. Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200 (2005).
Masuda, T. & Shiga, T. Chemorepulsion and cell adhesion molecules in patterning initial trajectories of sensory axons. Neurosci. Res. 51, 337–347 (2005).
Miller, L. E. et al. Increased prevalence of semaphorin 3C, a repellent of sympathetic nerve fibers, in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 50, 1156–1163 (2004).
Fassold, A. et al. Soluble neuropilin-2, a nerve repellent receptor, is increased in rheumatoid arthritis synovium and aggravates sympathetic fiber repulsion and arthritis. Arthritis Rheum. 60, 2892–2901 (2009).
Seki, T., Selby, J., Haupl, T. & Winchester, R. Use of differential subtraction method to identify genes that characterize the phenotype of cultured rheumatoid arthritis synoviocytes. Arthritis Rheum. 41, 1356–1364 (1998).
Ikeda, M., Hosoda, Y., Hirose, S., Okada, Y. & Ikeda, E. Expression of vascular endothelial growth factor isoforms and their receptors Flt-1, KDR, and neuropilin-1 in synovial tissues of rheumatoid arthritis. J. Pathol. 191, 426–433 (2000).
Matthies, A. M., Low, Q. E., Lingen, M. W. & DiPietro, L. A. Neuropilin-1 participates in wound angiogenesis. Am. J. Pathol. 160, 289–296 (2002).
Kigel, B., Varshavsky, A., Kessler, O. & Neufeld, G. Successful inhibition of tumor development by specific class-3 semaphorins is associated with expression of appropriate semaphorin receptors by tumor cells. PLoS ONE 3, e3287 (2008).
Kong, J. S. et al. Anti-neuropilin-1 peptide inhibition of synoviocyte survival, angiogenesis, and experimental arthritis. Arthritis Rheum. 62, 179–190 (2010).
Enomoto, H. et al. Vascular endothelial growth factor isoforms and their receptors are expressed in human osteoarthritic cartilage. Am. J. Pathol. 162, 171–181 (2003).
Gomez, C. et al. Expression of Semaphorin-3A and its receptors in endochondral ossification: potential role in skeletal development and innervation. Dev. Dyn. 234, 393–403 (2005).
Catalano, A. The neuroimmune semaphorin-3A reduces inflammation and progression of experimental autoimmune arthritis. J. Immunol. 185, 6373–6383 (2010).
Nico, B., Mangieri, D., Benagiano, V., Crivellato, E. & Ribatti, D. Nerve growth factor as an angiogenic factor. Microvasc. Res. 75, 135–141 (2008).
Raychaudhuri, S. K., Raychaudhuri, S. P., Weltman, H. & Farber, E. M. Effect of nerve growth factor on endothelial cell biology: proliferation and adherence molecule expression on human dermal microvascular endothelial cells. Arch. Dermatol. Res. 293, 291–295 (2001).
Cantarella, G. et al. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 16, 1307–1309 (2002).
Moser, K. V., Reindl, M., Blasig, I. & Humpel, C. Brain capillary endothelial cells proliferate in response to NGF, express NGF receptors and secrete NGF after inflammation. Brain Res. 1017, 53–60 (2004).
Steinle, J. J. & Granger, H. J. Nerve growth factor regulates human choroidal, but not retinal, endothelial cell migration and proliferation. Auton. Neurosci. 108, 57–62 (2003).
Tanaka, A., Wakita, U., Kambe, N., Iwasaki, T. & Matsuda, H. An autocrine function of nerve growth factor for cell cycle regulation of vascular endothelial cells. Biochem. Biophys. Res. Commun. 313, 1009–1014 (2004).
Rahbek, U. L., Dissing, S., Thomassen, C., Hansen, A. J. & Tritsaris, K. Nerve growth factor activates aorta endothelial cells causing PI3K/Akt- and ERK-dependent migration. Pflugers Arch. 450, 355–361 (2005).
Park, M. J. et al. Nerve growth factor induces endothelial cell invasion and cord formation by promoting matrix metalloproteinase-2 expression through the phosphatidylinositol 3-kinase/Akt signaling pathway and AP-2 transcription factor. J. Biol. Chem. 282, 30485–30496 (2007).
Lazarovici, P., Gazit, A., Staniszewska, I., Marcinkiewicz, C. & Lelkes, P. I. Nerve growth factor (NGF) promotes angiogenesis in the quail chorioallantoic membrane. Endothelium 13, 51–59 (2006).
Lazarovici, P., Marcinkiewicz, C. & Lelkes, P. I. Cross talk between the cardiovascular and nervous systems: neurotrophic effects of vascular endothelial growth factor (VEGF) and angiogenic effects of nerve growth factor (NGF)-implications in drug development. Curr. Pharm. Des. 12, 2609–2622 (2006).
Seo, K., Choi, J., Park, M. & Rhee, C. Angiogenesis effects of nerve growth factor (NGF) on rat corneas. J. Vet. Sci. 2, 125–130 (2001).
Calza, L., Giardino, L., Giuliani, A., Aloe, L. & Levi-Montalcini, R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc. Natl Acad. Sci. USA 98, 4160–4165 (2001).
Santos, P. M., Winterowd, J. G., Allen, G. G., Bothwell, M. A. & Rubel, E. W. Nerve growth factor: increased angiogenesis without improved nerve regeneration. Otolaryngol. Head Neck Surg. 105, 12–25 (1991).
Graiani, G. et al. Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of type 1 diabetic mice. Diabetologia 47, 1047–1054 (2004).
Liu, X. et al. Neuronal-driven angiogenesis: role of NGF in retinal neovascularization in an oxygen-induced retinopathy model. Invest. Ophthalmol. Vis. Sci. 51, 3749–3757 (2010).
Emanueli, C. et al. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation 106, 2257–2262 (2002).
Adriaenssens, E. et al. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 68, 346–351 (2008).
US National Library of Medicine. Phase III Study of docetaxel and ramucirumab or placebo in breast cancer. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. A study of paclitaxel with or without ramucirumab in metastatic gastric adenocarcinoma (RAINBOW). ClinicalTrials.gov [online] (2011).
US National Library of Medicine. A study of chemotherapy and ramucirumab vs. chemotherapy alone in second line non-small cell lung cancer patients who received prior first line platinum based chemotherapy ClinicalTrials.gov [online] (2012).
Strassle, B. W. et al. Inhibition of osteoclasts prevents cartilage loss and pain in a rat model of degenerative joint disease. Osteoarthritis Cartilage 18, 1319–1328 (2010).
Mapp., P. I., Walsh, D. A., Bowyer, J. & Maciewicz, R. A. Effects of a metalloproteinase inhibitor on osteochondral angiogenesis, chondropathy and pain behavior in a rat model of osteoarthritis. Osteoarthritis Cartilage 18, 593–600 (2010).
Moreau, M. et al. Tiludronate treatment improves structural changes and symptoms of osteoarthritis in the canine anterior cruciate ligament model. Arthritis Res. Ther. 13, R98 (2011).
Agnello, K. A., Trumble, T. N., Chambers, J. N., Seewald, W. & Budsberg, S. C. Effects of zoledronate on markers of bone metabolism and subchondral bone mineral density in dogs with experimentally induced cruciate-deficient osteoarthritis. Am. J. Vet. Res. 66, 1487–1495 (2005).
Jones, M. D. et al. In vivo microfocal computed tomography and micro-magnetic resonance imaging evaluation of antiresorptive and antiinflammatory drugs as preventive treatments of osteoarthritis in the rat. Arthritis Rheum. 62, 2726–2735 (2010).
Spector, T. D. et al. Effect of risedronate on joint structure and symptoms of knee osteoarthritis: results of the BRISK randomized, controlled trial [ISRCTN01928173]. Arthritis Res. Ther. 7, R625–R633 (2005).
Bingham, C. O. 3rd et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 54, 3494–3507 (2006).
Laslett, L. et al. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2011-200970
Lane, N. E. et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N. Engl. J. Med. 363, 1521–1531 (2010).
Dixon, W. & Felson, D. T. Is anti-TNF therapy safer than previously thought? JAMA 306, 2380–2381 (2011).
Jeng, L., Olsen, B. R. & Spector, M. Engineering endostatin-producing cartilaginous constructs for cartilage repair using nonviral transfection of chondrocyte-seeded and mesenchymal-stem-cell-seeded collagen scaffolds. Tissue Eng. Part A 16, 3011–3021 (2010).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to researching data for the article and writing, editing and reviewing the manuscript.
Corresponding author
Ethics declarations
Competing interests
D. A. Walsh declares that he has acted as a consultant for Pfizer. P. I. Mapp declares no competing interests.
Rights and permissions
About this article
Cite this article
Mapp, P., Walsh, D. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol 8, 390–398 (2012). https://doi.org/10.1038/nrrheum.2012.80
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2012.80
This article is cited by
-
ADAM8 silencing suppresses the migration and invasion of fibroblast-like synoviocytes via FSCN1/MAPK cascade in osteoarthritis
Arthritis Research & Therapy (2024)
-
Combined genicular artery embolization and genicular nerve block to treat chronic pain following total knee arthroplasty
CVIR Endovascular (2024)
-
Potential therapeutic strategies for osteoarthritis via CRISPR/Cas9 mediated gene editing
Reviews in Endocrine and Metabolic Disorders (2024)
-
Causal association between cardiovascular proteins and membranous nephropathy: a bidirectional Mendelian randomization
International Urology and Nephrology (2024)
-
Nav1.7 as a chondrocyte regulator and therapeutic target for osteoarthritis
Nature (2024)