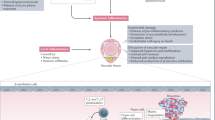
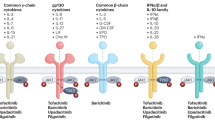
Abstract
Biologic therapies have revolutionized the treatment of rheumatic diseases in the past decade. As with any drugs, however, a variety of important safety concerns affect the choice and use of these agents. Several issues, such as the risk of infection, malignancy, or administration reactions, apply to all of these compounds, although some conditions that affect patient selection and management within these categories seem to be specific to particular biologic treatments. Other safety concerns with biologic agents, such as congestive heart failure, demyelinating disease, and hyperlipidemia, are associated with individual agents. Despite all these concerns, the therapeutic indices for biologic agents remain fairly high in relation to non-biologic DMARDs. Available safety data for all biologic agents approved for the treatment of rheumatoid arthritis are reviewed in this manuscript. With careful patient selection and appropriate vigilance on the part of treating physicians and other care providers, these compounds can be safely integrated into the therapeutic plan.
Key Points
-
Although randomized, controlled trials provide important initial information about the safety of new therapeutic agents, post-marketing data are often necessary to understand the true risk of specific adverse events
-
Infections remain the greatest safety concern with biologic therapies; no single agent carries a clearly reduced overall risk of infection in comparison with the others
-
The risk of specific infections, such as tuberculosis, seems to be higher with some biologic agents than with others
-
Screening for latent tuberculosis in all patients can reduce the risk of reactivation of this infection with TNF inhibitor therapy
-
Whereas the overall risk of solid malignancies does not seem to increase with biologic therapy, the risk of non-melanoma skin cancer is increased with TNF inhibitor therapy
-
TNF inhibitors may be used during pregnancy if clinically indicated; insufficient data guide the potential use of other biologic agents during pregnancy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Aggarwal, B. B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3, 745–756 (2003).
Baum, J. Infection in rheumatoid arthritis. Arthritis Rheum. 14, 135–137 (1971).
Bongartz, T. et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 295, 2275–2285 (2006).
Singh, J. A. et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database of Systematic Reviews, Issue 2. Art. No.: CD008794 doi:10.1002/14651858.CD008794.pub2 (2011).
Askling, J. et al. Time-dependent increase in risk of hospitalisation with infection among Swedish RA patients treated with TNF antagonists. Ann. Rheum. Dis. 66, 1339–1344 (2007).
Galloway, J. B. et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 50, 124–131 (2011).
Greenberg, J. D. et al. Association of methotrexate and tumour necrosis factor antagonists with risk of infectious outcomes including opportunistic infections in the CORRONA registry. Ann. Rheum. Dis. 69, 380–386 (2010).
Au, K. et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann. Rheum. Dis. 70, 785–791 (2011).
Wolfe, F., Caplan, L. & Michaud, K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum. 54, 628–634 (2006).
Emery, P. et al. Golimumab, a human anti-tumor necrosis factor α monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 60, 2272–2283 (2009).
Keystone, E. et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 58, 3319–3329 (2008).
Keystone, E. C. et al. Golimumab, a human antibody to tumour necrosis factor α given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann. Rheum. Dis. 68, 789–796 (2009).
Smolen, J. et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann. Rheum. Dis. 68, 797–804 (2009).
Smolen, J. S. et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet 374, 210–221 (2009).
Fleischmann, R. M. Addressing the safety of anakinra in patients with rheumatoid arthritis. Rheumatology (Oxford) 42 (Suppl. 2), ii29–ii35 (2003).
Salliot, C., Dougados, M. & Gossec, L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann. Rheum. Dis. 68, 25–32 (2009).
Mertens, M. & Singh, J. A. Anakinra for rheumatoid arthritis: a systematic review. J. Rheumatol. 36, 1118–1125 (2009).
Swart, J. F., Barug, D., Mohlmann, M. & Wulffraat, N. M. The efficacy and safety of interleukin-1-receptor antagonist anakinra in the treatment of systemic juvenile idiopathic arthritis. Expert Opin. Biol. Ther. 10, 1743–1752 (2010).
Nishimoto, N. et al. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann. Rheum. Dis. 68, 1580–1584 (2009).
Smolen, J. S. et al. Safety of tocilizumab in patients with rheumatoid arthritis: pooled analysis of five phase 3 clinical trials [abstract 1669]. Arthritis Rheum. 54 (Suppl.), S784 (2008).
Hoshi, D. et al. Incidence of serious respiratory infections in patients with rheumatoid arthritis treated with tocilizumab. Mod. Rheumatol. http://dx.doi.org/10.1007/s10165-011-0488-6.
Weinblatt, M. et al. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: A one-year randomized, placebo-controlled study. Arthritis Rheum. 54, 2807–2816 (2006).
Orencia® (abatacept) full prescribing information. (Bristol-Meyers Squibb, 2011).
Westhovens, R. et al. Safety and efficacy of the selective costimulation modulator abatacept in patients with rheumatoid arthritis receiving background methotrexate: a 5-year extended phase IIB study. J. Rheumatol. 36, 736–742 (2009).
Weinblatt, M. et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann. Rheum. Dis. 66, 228–234 (2007).
Schiff, M. et al. The 6-month safety and efficacy of abatacept in patients with rheumatoid arthritis who underwent a washout after anti-tumour necrosis factor therapy or were directly switched to abatacept: the ARRIVE trial. Ann. Rheum. Dis. 68, 1708–1714 (2009).
Mohrbacher, A. B cell non-Hodgkin's lymphoma: rituximab safety experience. Arthritis Res. Ther. 7 (Suppl. 3), S19–S25 (2005).
Cohen, S. B. et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 54, 2793–2806 (2006).
Emery, P. et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 54, 1390–1400 (2006).
van Vollenhoven, R. F. et al. Longterm safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J. Rheumatol. 37, 558–567 (2010).
Keystone, E. et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum. 56, 3896–3908 (2007).
Keane, J. et al. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N. Engl. J. Med. 345, 1098–1104 (2001).
Wallis, R. S., Broder, M. S., Wong, J. Y., Hanson, M. E. & Beenhouwer, D. O. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin. Infect. Dis. 38, 1261–1265 (2004).
Askling, J. et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum. 52, 1986–1992 (2005).
Gomez-Reino, J. J., Carmona, L., Valverde, V. R., Mola, E. M. & Montero, M. D. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 48, 2122–2127 (2003).
Tubach, F. et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: The three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 60, 1884–1894 (2009).
Carmona, L. et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 52, 1766–1772 (2005).
Winthrop, K. L., Chang, E., Yamashita, S., Iademarco, M. F. & LoBue, P. A. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-α therapy. Emerg. Infect. Dis. 15, 1556–1561 (2009).
Bauman, S. K., Huffnagle, G. B. & Murphy, J. W. Effects of tumor necrosis factor α on dendritic cell accumulation in lymph nodes draining the immunization site and the impact on the anticryptococcal cell-mediated immune response. Infect. Immun. 71, 68–74 (2003).
Cenci, E. et al. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J. Infect. Dis. 178, 1750–1760 (1998).
Cox, R. A. & Magee, D. M. Production of tumor necrosis factor α, interleukin-1 α, and interleukin-6 during murine coccidioidomycosis. Infect. Immun. 63, 4178–4180 (1995).
Bergstrom, L. et al. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor α antagonists. Arthritis Rheum. 50, 1959–1966 (2004).
Lee, J. H. et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor α antagonists infliximab and etanercept. Arthritis Rheum. 46, 2565–2570 (2002).
Kalyoncu, U. et al. Pneumocystis carinii pneumonia in a rheumatoid arthritis patient treated with adalimumab. Scand. J. Infect. Dis. 39, 475–478 (2007).
Kaur, N. & Mahl, T. C. Pneumocystis jiroveci (carinii) pneumonia after infliximab therapy: a review of 84 cases. Dig. Dis. Sci. 52, 1481–1484 (2007).
Lahiff, C., Khiaron, O. B., Nolan, N. & Chadwick, G. A. Pneumocystis carinii pneumonia in a patient on etanercept for psoriatic arthritis. Ir. J. Med. Sci. 176, 309–311 (2007).
Mori, S., Cho, I., Ichiyasu, H. & Sugimoto, M. Asymptomatic carriage of Pneumocystis jiroveci in elderly patients with rheumatoid arthritis in Japan: a possible association between colonization and development of Pneumocystis jiroveci pneumonia during low-dose MTX therapy. Mod. Rheumatol. 18, 240–246 (2008).
Fleischmann, R. M. et al. Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: a large, international, multicenter, placebo-controlled trial. Arthritis Rheum. 48, 927–934 (2003).
van Vollenhoven, R. et al. Safety of tocilizumab in patients with rheumatoid arthritis: analysis of median of 2.6 years of treatment in long-term extension studies [abstract 172]. Ann. Rheum. Dis. 69 (Suppl. 3), 544 (2010).
Simon, T. A. et al. Infections requiring hospitalization in the abatacept clinical development program: an epidemiological assessment. Arthritis Res. Ther. 12, R67 (2010).
Bigbee, C. L. et al. Abatacept treatment does not exacerbate chronic Mycobacterium tuberculosis infection in mice. Arthritis Rheum. 56, 2557–2565 (2007).
Teichmann, L. L. et al. Fatal Pneumocystis pneumonia following rituximab administration for rheumatoid arthritis. Rheumatology (Oxford) 47, 1256–1257 (2008).
Gea-Banacloche, J. C. Rituximab-associated infections. Semin. Hematol. 47, 187–198 (2010).
Actemra® (tocilizumab) full prescribing information. (Genentech, 2011).
Garcia-Doval, I. et al. Incidence and risk of hospitalisation due to shingles and chickenpox in patients with rheumatic diseases treated with TNF antagonists. Ann. Rheum. Dis. 69, 1751–1755 (2010).
Strangfeld, A. et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-α agents. JAMA 301, 737–744 (2009).
Kim, Y. J. et al. Possible reactivation of potential hepatitis B virus occult infection by tumor necrosis factor- α blocker in the treatment of rheumatic diseases. J. Rheumatol. 37, 346–350 (2010).
Carroll, M. B. & Bond, M. I. Use of tumor necrosis factor- α inhibitors in patients with chronic hepatitis B infection. Semin. Arthritis Rheum. 38, 208–217 (2008).
Evens, A. M. et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann. Oncol. 22, 1170–1180 (2011).
Padgett, B. L. & Walker, D. L. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J. Infect. Dis. 127, 467–470 (1973).
Carson, K. R. et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 113, 4834–4840 (2009).
Bingham, C. O. 3rd et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 62, 64–74 (2010).
Gelinck, L. B. et al. The effect of anti-tumour necrosis factor α treatment on the antibody response to influenza vaccination. Ann. Rheum. Dis. 67, 713–716 (2008).
Salemi, S. et al. Influenza vaccine administration in rheumatoid arthritis patients under treatment with TNFα blockers: safety and immunogenicity. Clin. Immunol. 134, 113–120 (2010).
Saag, K. G. et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 59, 762–784 (2008).
Cheuk, D. K., Chiang, A. K., Lee, T. L., Chan, G. C. & Ha, S. Y. Vaccines for prophylaxis of viral infections in patients with hematological malignancies. Cochrane Database of Systematic Reviews, Issue 3. Art No.: CD006505 doi:10.1002/14651858.CD006505.pub2 (2011).
Ekstrom, K. et al. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum. 48, 963–970 (2003).
Ramsey-Goldman, R. et al. Increased risk of malignancy in patients with systemic lupus erythematosus. J. Investig. Med. 46, 217–222 (1998).
Sigurgeirsson, B., Lindelof, B., Edhag, O. & Allander, E. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N. Engl. J. Med. 326, 363–367 (1992).
Baecklund, E. et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 54, 692–701 (2006).
Mariette, X. et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann. Rheum. Dis. http://dx.doi.org/10.1136/ard.2010.149419.
Wolfe, F. & Michaud, K. Lymphoma in rheumatoid arthritis: the effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 50, 1740–1751 (2004).
Askling, J. et al. Anti-tumour necrosis factor therapy in rheumatoid arthritis and risk of malignant lymphomas: relative risks and time trends in the Swedish Biologics Register. Ann. Rheum. Dis. 68, 648–653 (2009).
Setoguchi, S. et al. Tumor necrosis factor alpha antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum. 54, 2757–2764 (2006).
Mackey, A. C., Green, L., Leptak, C. & Avigan, M. Hepatosplenic T-cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease: update. J. Pediatr. Gastroenterol. Nutr. 48, 386–388 (2009).
Simponi® (golimumab) full prescribing information (Janssen Biotech, 2011).
Remicade® (infliximab) full prescribing information (Janssen Biotech, 2011).
Cimzia® (certolizumab) full prescribing information (UCB, 2011).
Humira® (adalimumab) full prescribing information (Abbott Immunology, 2011).
Enbrel® (etanercept) full prescribing information (Amgen, 2011).
Wolfe, F. & Michaud, K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 56, 2886–2895 (2007).
Amari, W. et al. Risk of non-melanoma skin cancer in a national cohort of veterans with rheumatoid arthritis. Rheumatology (Oxford) 50, 1431–1439 (2011).
Chakravarty, E. F., Michaud, K. & Wolfe, F. Skin cancer, rheumatoid arthritis, and tumor necrosis factor inhibitors. J. Rheumatol. 32, 2130–2135 (2005).
Simon, T. A. et al. Malignancies in the rheumatoid arthritis abatacept clinical development programme: an epidemiological assessment. Ann. Rheum. Dis. 68, 1819–1826 (2009).
Dixon, W. G. et al. Influence of anti-tumor necrosis factor therapy on cancer incidence in patients with rheumatoid arthritis who have had a prior malignancy: results from the British Society for Rheumatology Biologics Register. Arthritis Care Res. (Hoboken) 62, 755–763 (2010).
Maini, R. et al. Infliximab (chimeric anti-tumour necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 354, 1932–1939 (1999).
van de Putte, L. B. et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann. Rheum. Dis. 63, 508–516 (2004).
Ramos-Casals, M. et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 86, 242–251 (2007).
Smitten, A. L., Qi, K., Simon, T. A. & Becker, J. C. Autoimmune adverse events in the abatacept RA clinical development program: a safety analysis with >10, 000 person-years of exposure [abstract 1673]. Arthritis Rheum. 58 (Suppl.), S786 (2008).
Davaine, A. C. et al. Cutaneous events during treatment of chronic inflammatory joint disorders with anti-tumour necrosis factor α: a cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 22, 1471–1477 (2008).
Goncalves, D. P., Laurindo, I. & Scheinberg, M. A. The appearance of pustular psoriasis during antitumor necrosis factor therapy. J. Clin. Rheumatol. 12, 262 (2006).
Lee, H. H. et al. Cutaneous side-effects in patients with rheumatic diseases during application of tumour necrosis factor-α antagonists. Br. J. Dermatol. 156, 486–491 (2007).
Dass, S., Vital, E. M. & Emery, P. Development of psoriasis after B-cell depletion with rituximab. Arthritis Rheum. 56, 2715–2718 (2007).
Sibilia, J. & Westhovens, R. Safety of T-cell co-stimulation modulation with abatacept in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 25 (Suppl. 46), S46–S56 (2007).
Pontikaki, I. et al. Skin manifestations induced by TNF-α inhibitors in juvenile idiopathic arthritis. Clin. Rev. Allergy Immunol. http://dx.doi.org/10.1007/s12016-011-8262-2.
Kerbleski, J. F. & Gottlieb, A. B. Dermatological complications and safety of anti-TNF treatments. Gut 58, 1033–1039 (2009).
Bannwarth, B. & Richez, C. Clinical safety of tocilizumab in rheumatoid arthritis. Expert Opin. Drug Saf. 10, 123–131 (2011).
Bartelds, G. M. et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 305, 1460–1468 (2011).
Haraoui, B., Cameron, L., Ouellet, M. & White, B. Anti-infliximab antibodies in patients with rheumatoid arthritis who require higher doses of infliximab to achieve or maintain a clinical response. J. Rheumatol. 33, 31–36 (2006).
Khraishi, M., Russell, A. & Olszynski, W. P. Safety profile of abatacept in rheumatoid arthritis: a review. Clin. Ther. 32, 1855–1870 (2010).
Zeltser, R. et al. Clinical, histological, and immunophenotypic characteristics of injection site reactions associated with etanercept: a recombinant tumor necrosis factor α receptor: Fc fusion protein. Arch. Dermatol. 137, 893–899 (2001).
Paltiel, M. et al. Immediate type I hypersensitivity response implicated in worsening injection site reactions to adalimumab. Arch. Dermatol. 144, 1190–1194 (2008).
Kay, J. et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 58, 964–975 (2008).
Borras-Blasco, J., Navarro-Ruiz, A., Borras, C. & Castera, E. Adverse cutaneous reactions induced by TNF-α antagonist therapy. South. Med. J. 102, 1133–1140 (2009).
Wolfe, F. et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 37, 481–494 (1994).
Levine, B., Kalman, J., Mayer, L., Fillit, H. M. & Packer, M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 323, 236–241 (1990).
Yokoyama, T. et al. Cellular basis for the negative inotropic effects of tumor necrosis factor-α in the adult mammalian heart. J. Clin. Invest. 92, 2303–2312 (1993).
Sarzi-Puttini, P., Atzeni, F., Shoenfeld, Y. & Ferraccioli, G. TNF-α, rheumatoid arthritis, and heart failure: a rheumatological dilemma. Autoimmun. Rev. 4, 153–161 (2005).
Symmons, D. P. & Gabriel, S. E. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat. Rev. Rheumatol. 7, 399–408 (2011).
Nishimoto, N., Ito, K. & Takagi, N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: meta-analysis of six initial trials and five long-term extensions. Mod. Rheumatol. 20, 222–232 (2010).
Nuki, G., Bresnihan, B., Bear, M. B. & McCabe, D. Long-term safety and maintenance of clinical improvement following treatment with anakinra (recombinant human interleukin-1 receptor antagonist) in patients with rheumatoid arthritis: extension phase of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 46, 2838–2846 (2002).
van Vollenhoven, R. et al. Gastrointestinal safety in patients with rheumatoid arthritis treated with tocilizumab: data from Roche clinical trials [abstract 1613]. Arthritis Rheum. 60 (Suppl.), S602 (2009).
Gottenberg, J. E. et al. Risk factors for severe infections in patients with rheumatoid arthritis treated with rituximab in the autoimmunity and rituximab registry. Arthritis Rheum. 62, 2625–2632 (2010).
Wiendl, H. & Hohlfeld, R. Therapeutic approaches in multiple sclerosis: lessons from failed and interrupted treatment trials. BioDrugs 16, 183–200 (2002).
Mohan, N. et al. Demyelination occurring during anti-tumor necrosis factor α therapy for inflammatory arthritides. Arthritis Rheum. 44, 2862–2869 (2001).
Fromont, A., De Seze, J., Fleury, M. C., Maillefert, J. F. & Moreau, T. Inflammatory demyelinating events following treatment with anti-tumor necrosis factor. Cytokine 45, 55–57 (2009).
Li, S. Y., Birnbaum, A. D. & Goldstein, D. A. Optic neuritis associated with adalimumab in the treatment of uveitis. Ocul. Immunol. Inflamm. 18, 475–481 (2010).
Carter, J. D., Valeriano, J. & Vasey, F. B. Tumor necrosis factor-α inhibition and VATER association: a causal relationship. J. Rheumatol. 33, 1014–1017 (2006).
Ali, Y. M., Kuriya, B., Orozco, C., Cush, J. J. & Keystone, E. C. Can tumor necrosis factor inhibitors be safely used in pregnancy? J. Rheumatol. 37, 9–17 (2010).
Carter, J. D., Ladhani, A., Ricca, L. R., Valeriano, J. & Vasey, F. B. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J. Rheumatol. 36, 635–641 (2009).
Osting, V. C. & Carter, J. D. A safety assessment of tumor necrosis factor antagonists during pregnancy. Expert Opin. Drug Saf. 9, 421–429 (2010).
Roux, C. H., Brocq, O., Breuil, V., Albert, C. & Euller-Ziegler, L. Pregnancy in rheumatology patients exposed to anti-tumour necrosis factor (TNF)-α therapy. Rheumatology (Oxford) 46, 695–698 (2007).
Chambers, C. D., Tutuncu, Z. N., Johnson, D. & Jones, K. L. Human pregnancy safety for agents used to treat rheumatoid arthritis: adequacy of available information and strategies for developing post-marketing data. Arthritis Res. Ther. 8, 215 (2006).
Chakravarty, E. F., Murray, E. R., Kelman, A. & Farmer, P. Pregnancy outcomes after maternal exposure to rituximab. Blood 117, 1499–1506 (2011).
Briggs, G. G., Freeman, R. F. & Yaffe, S. J. Drugs in Pregnancy and Lactation, (Williams & Wilkins, Philadelphia, 2008).
Listing, J. et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 52, 3403–3412 (2005).
Acknowledgements
C. P. Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape, LLC-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Contributions
R. S. Woodrick and E. M. Ruderman contributed equally to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
E. M. Ruderman is a consultant for Amgen, Abbott, Roche, Wyeth/Pfizer and UCB, and has received research support from Abbott, Centocor and Roche. R. Woodrick declares no competing interests.
Rights and permissions
About this article
Cite this article
Woodrick, R., Ruderman, E. Safety of biologic therapy in rheumatoid arthritis. Nat Rev Rheumatol 7, 639–652 (2011). https://doi.org/10.1038/nrrheum.2011.145
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2011.145
This article is cited by
-
Safety of Biologics Approved for the Treatment of Rheumatoid Arthritis and Other Autoimmune Diseases: A Disproportionality Analysis from the FDA Adverse Event Reporting System (FAERS)
BioDrugs (2018)
-
Biologic drugs and arrhythmic risk in chronic inflammatory arthritis: the good and the bad
Immunologic Research (2017)
-
Targeting IL-17 in autoimmunity and inflammation
Archives of Pharmacal Research (2016)
-
Calcium channels: the potential therapeutic targets for inflammatory bone destruction of rheumatoid arthritis
Inflammation Research (2016)
-
Successful resumption of tocilizumab for rheumatoid arthritis after resection of a pulmonary Mycobacterium avium complex lesion: a case report
BMC Pulmonary Medicine (2015)