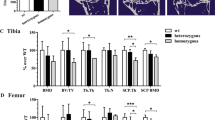
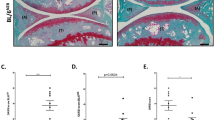
Abstract
Mitochondria are important regulators of cellular function and survival that may have a key role in aging-related diseases. Mitochondrial DNA (mtDNA) mutations and oxidative stresses are known to contribute to aging-related changes. Osteoarthritis (OA) is an aging-associated rheumatic disease characterized by articular cartilage degradation and elevated chondrocyte mortality. Articular cartilage chondrocytes survive and maintain tissue integrity in an avascular, low-oxygen environment. Recent ex vivo studies have reported mitochondrial dysfunction in human OA chondrocytes, and analyses of mitochondrial electron transport chain activity in these cells show decreased activity of Complexes I, II and III compared to normal chondrocytes. This mitochondrial dysfunction may affect several pathways that have been implicated in cartilage degradation, including oxidative stress, defective chondrocyte biosynthesis and growth responses, increased cytokine-induced chondrocyte inflammation and matrix catabolism, cartilage matrix calcification, and increased chondrocyte apoptosis. Mitochondrial dysfunction in OA chondrocytes may derive from somatic mutations in the mtDNA or from the direct effects of proinflammatory mediators such as cytokines, prostaglandins, reactive oxygen species and nitric oxide. Polymorphisms in mtDNA may become useful as biomarkers for the diagnosis and prognosis of OA, and modulation of serum biomarkers by mtDNA haplogroups supports the concept that mtDNA haplogroups may define specific OA phenotypes in the complex OA process.
Key Points
-
Mitochondrial functions, including mitochondrial respiratory chain (MRC) activity and ATP synthesis, are altered in osteoarthritis (OA) chondrocytes
-
Mitochondrial dysfunction may influence several of the specific pathways involved in OA pathology, including oxidative stress, chondrocyte apoptosis, cytokine-induced chondrocyte inflammation and matrix catabolism, and calcification of the cartilage matrix
-
OA chondrocyte mitochondrial dysfunction may originate from somatic mutations in the mitochondrial DNA (mtDNA) or from the direct effects of proinflammatory cytokines, prostaglandins, reactive oxygen species and nitric oxide on the MRC and ATP synthesis
-
mtDNA haplogroups may serve as useful biomarkers for the diagnosis or prognosis of OA, and might define distinct, specific OA phenotypes with different levels of serum OA biomarkers
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Spector, T. D. Epidemiology of the rheumatic diseases. Curr. Opin. Rheumatol. 5, 132–137 (1993).
Lotz, M. et al. Cytokine regulation of chondrocyte functions. J. Rheumatol. Suppl. 43, 104–108 (1995).
Maneiro, E. et al. Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann. Rheum. Dis. 64, 388–395 (2005).
Afonso, V., Champy, R., Mitrovic, D., Collin, P. & Lomri, A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine 74, 324–329 (2007).
Henrotin, Y., Blanco, F. J., Aigner, T. & Kurz, B. The significance of oxidative stress in articular cartilage ageing and degradation. Curr. Rheumatol. Rev. 3, 261–274 (2007).
Henze, K. & Martin, W. Evolutionary biology: essence of mitochondria. Nature 426, 127–128 (2003).
Voet, D., Voet, J. G. & Pratt, C. W. Fundamentals of Biochemistry: Life at the Molecular Level 3rd edn (John Wiley & Sons, 2009).
Alberts, B. et al. Molecular Biology of the Cell 4th edn (Garland Science, New York, 2002).
Ross, P. L. et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell. Proteomics 3, 1154–1169 (2004).
Chinnery, P. F., Howell, N., Andrews, R. M. & Turnbull, D. M. Clinical mitochondrial genetics. J. Med. Genet. 36, 425–436 (1999).
Marcus, R. E. The effect of low oxygen concentration on growth, glycolysis, and sulfate incorporation by articular chondrocytes in monolayer culture. Arthritis Rheum. 16, 646–656 (1973).
Oegema, T. R. J. & Thompson, R. C. in Articular Cartilage Biochemistry (eds Kuettner, K. et al.) 257–271 (Raven Press, New York, 1986).
Shapiro, I. M., Tokuoka, T. & Silverton, S. F. in Cartilage: Molecular Aspects (eds Hall, B. K. & Newman, S. A.) 97–130 (CRC Press, Boca Raton, FL, 1991).
Maroudas, A. in Adult Articular Cartilage (ed. Freeman, M. A. R.) 131–170 (Grune & Stratton, New York, 1973).
Falchuk, K. H., Goetzl, E. J. & Kulka, J. P. Respiratory gases of synovial fluids. An approach to synovial tissue circulatory-metabolic imbalance in rheumatoid arthritis. Am. J. Med. 49, 223–231 (1970).
Lund-Oleson, K. Oxygen tension in synovial fluids. Arthritis Rheum. 13, 769–776 (1970).
Zhou, S., Cui, Z. & Urban, J. P. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage–bone interface: a modeling study. Arthritis Rheum. 50, 3915–3924 (2004).
Yamamoto, T. & Gay, C. V. Ultrastructural analysis of cytochrome oxidase in chick epiphyseal growth plate cartilage. J. Histochem. Cytochem. 36, 1161–1166 (1988).
Henrotin, Y., Kurz, B. & Aigner, T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage 13, 643–654 (2005).
Maneiro, E. et al. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Rheum. 48, 700–708 (2003).
Johnson, K. et al. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheum. 43, 1560–1570 (2000).
Terkeltaub, R., Johnson, K., Murphy, A. & Ghosh, S. Invited review: the mitochondrion in osteoarthritis. Mitochondrion 1, 301–319 (2002).
Ruiz-Romero, C. et al. Hypoxia conditions differentially modulate human normal and osteoarthritic chondrocyte proteomes. J. Proteome Res. 9, 3035–3045 (2010).
Tomita, M., Sato, E. F., Nishikawa, M., Yamano, Y. & Inoue, M. Nitric oxide regulates mitochondrial respiration and functions of articular chondrocytes. Arthritis Rheum. 44, 96–104 (2001).
Turrens, J. F. Mitochondrial formation of reactive oxygen species. J. Physiol. 552, 335–344 (2003).
Cillero-Pastor, B. et al. Mitochondrial dysfunction activates cyclooxygenase 2 expression in cultured normal human chondrocytes. Arthritis Rheum. 58, 2409–2419 (2008).
Caramés, B. et al. Inhibition of mitochondrial respiratory chain induces an inflammatory response in human articular chondrocytes. Ann. Rheum. Dis. 64 (Suppl. 3), 142–143 (2005).
Lopez-Armada, M. J. et al. Mitochondrial activity is modulated by TNFα and IL-1β in normal human chondrocyte cells. Osteoarthritis Cartilage 14, 1011–1022 (2006).
Landis, W. J. Application of electron probe X-ray microanalysis to calcification studies of bone and cartilage. Scan. Electron Microsc. 2, 555–570 (1979).
Shapiro, I. M. et al. Initiation of endochondral calcification is related to changes in the redox state of hypertrophic chondrocytes. Science 217, 950–952 (1982).
Stockwell, R. A. The cell density of human articular and costal cartilage. J. Anat. 101, 753–763 (1967).
Blanco, F., Guitian, R., Vázquez-Martul, E., de Toro, F. & Galdo, F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 41, 284–289 (1998).
Kim, H. A., Lee, Y. J., Seong, S. C., Choe, K. W. & Song, Y. W. Apoptotic chondrocyte death in human osteoarthritis. J. Rheumatol. 27, 455–462 (2000).
Hashimoto, S., Ochs, R. L., Komiya, S. & Lotz, M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 41, 1632–1638 (1998).
Aigner, T. et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 44, 1304–1312 (2001).
Aigner, T. & Kim, H. A. Apoptosis and cellular vitality: issues in osteoarthritic cartilage degeneration. Arthritis Rheum. 46, 1986–1996 (2002).
Roach, H. I., Aigner, T. & Kouri, J. B. Chondroptosis: a variant of apoptotic cell death in chondrocytes? Apoptosis 9, 265–277 (2004).
Kim, H. A. & Blanco, F. J. Cell death and apoptosis in osteoarthritic cartilage. Curr. Drug Targets 8, 333–345 (2007).
Torroni, A. et al. Classification of European mtDNAs from an analysis of three European populations. Genetics 144, 1835–1850 (1996).
Lin, M. T. & Beal, M. F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 (2006).
Taylor, S. W. et al. Characterization of the human heart mitochondrial proteome. Nat. Biotechnol. 21, 281–286 (2003).
Grishko, V. I., Ho, R., Wilson, G. L. & Pearsall, A. W. 4th. Diminished mitochondrial DNA integrity and repair capacity in OA chondrocytes. Osteoarthritis Cartilage 17, 107–113 (2009).
Chang, M. C. et al. Accumulaton of mitochondrial DNA with 4977-bp deletion in knee cartilage—an association with idiopathic osteoarthritis. Osteoarthritis Cartilage 13, 1004–1011 (2005).
Ruiz-Romero, C. et al. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: a decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol. Cell. Proteomics 8, 172–189 (2009).
Aigner, T. et al. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 54, 3533–3547 (2006).
Rachek, L. I., Grishko, V. I., Ledoux, S. P. & Wilson, G. L. Role of nitric oxide-induced mtDNA damage in mitochondrial dysfunction and apoptosis. Free Radic. Biol. Med. 40, 754–762 (2006).
Del Carlo, M. Jr & Loeser, R. F. Nitric oxide-mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum. 46, 394–403 (2002).
Whiteman, M., Rose, P., Siau, J. L. & Halliwell, B. Nitrite-mediated protection against hypochlorous acid-induced chondrocyte toxicity: a novel cytoprotective role of nitric oxide in the inflamed joint? Arthritis Rheum. 48, 3140–3150 (2003).
Kuhn, K., D'Lima, D. D., Hashimoto, S. & Lotz, M. Cell death in cartilage. Osteoarthritis Cartilage 12, 1–16 (2004).
Carlo, M. D. Jr & Loeser, R. F. Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis Rheum. 48, 3419–3430 (2003).
Whiteman, M. et al. Peroxynitrite mediates calcium-dependent mitochondrial dysfunction and cell death via activation of calpains. FASEB J. 18, 1395–1397 (2004).
Kim, J. et al. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthritis Cartilage 18, 424–432 (2010).
Dave, M. et al. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 58, 2786–2797 (2008).
Johnson, K. et al. Mediation of spontaneous knee osteoarthritis by progressive chondrocyte ATP depletion in Hartley guinea pigs. Arthritis Rheum. 50, 1216–1225 (2004).
van der Walt, J. M. et al. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am. J. Hum. Genet. 72, 804–811 (2003).
Niemi, A. K. et al. Mitochondrial DNA polymorphisms associated with longevity in a Finnish population. Hum. Genet. 112, 29–33 (2003).
Rego-Perez, I., Fernandez-Moreno, M., Fernandez-Lopez, C., Arenas, J. & Blanco, F. J. Mitochondrial DNA haplogroups: role in the prevalence and severity of knee osteoarthritis. Arthritis Rheum. 58, 2387–2396 (2008).
Rego, I. et al. Role of European mitochondrial DNA haplogroups in the prevalence of hip osteoarthritis in Galicia, Northern Spain. Ann. Rheum. Dis. 69, 210–213 (2010).
Rego-Pérez, I. et al. Mitochondrial DNA haplogroups modulate the serum levels of biomarkers in patients with osteoarthritis. Ann. Rheum. Dis. 69, 910–917 (2010).
Wallace, D. C. Mitochondrial diseases in man and mouse. Science 283, 1482–1488 (1999).
Mishmar, D. et al. Natural selection shaped regional mtDNA variation in humans. Proc. Natl Acad. Sci. USA 100, 171–176 (2003).
Wallace, D. C., Ruiz-Pesini, E. & Mishmar, D. mtDNA variation, climatic adaptation, degenerative diseases, and longevity. Cold Spring Harb. Symp. Quant. Biol. 68, 479–486 (2003).
Ruiz-Pesini, E., Mishmar, D., Brandon, M., Procaccio, V. & Wallace, D. C. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science 303, 223–226 (2004).
De Benedictis, G. et al. Mitochondrial DNA inherited variants are associated with successful aging and longevity in humans. FASEB J. 13, 1532–1536 (1999).
Ross, O. A. et al. Mitochondrial DNA polymorphism: its role in longevity of the Irish population. Exp. Gerontol. 36, 1161–1178 (2001).
Wolf, B. B. & Green, D. R. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J. Biol. Chem. 274, 20049–20052 (1999).
Brunner, T. & Mueller, C. Apoptosis in disease: about shortage and excess. Essays Biochem. 39, 119–130 (2003).
Pelletier, J. P., Fernandes, J. C., Jovanovic, D. V., Reboul, P. & Martel-Pelletier, J. Chondrocyte death in experimental osteoarthritis is mediated by MEK 1/2 and p38 pathways: role of cyclooxygenase-2 and inducible nitric oxide synthase. J. Rheumatol. 28, 2509–2519 (2001).
D'Lima, D. D., Hashimoto, S., Chen, P. C., Colwell, C. W. Jr & Lotz, M. K. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage 9, 712–719 (2001).
Huser, C. A., Peacock, M. & Davies, M. E. Inhibition of caspase-9 reduces chondrocyte apoptosis and proteoglycan loss following mechanical trauma. Osteoarthritis Cartilage 14, 1002–1010 (2006).
Feng, L., Precht, P., Balakir, R. & Horton, W. E. Jr. Evidence of a direct role for Bcl-2 in the regulation of articular chondrocyte apoptosis under the conditions of serum withdrawal and retinoic acid treatment. J. Cell. Biochem. 71, 302–309 (1998).
Malicev, E., Woyniak, G., Knezevic, M., Radosavljevic, D. & Jeras, M. Vitamin C induced apoptosis in human articular chondrocytes. Pflugers Arch. 440 (5 Suppl.), R46–R48 (2000).
Venezian, R., Shenker, B. J., Datar, S. & Leboy, P. S. Modulation of chondrocyte proliferation by ascorbic acid and BMP-2. J. Cell. Physiol. 174, 331–341 (1998).
Kraus, V. B. et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 50, 1822–1831 (2004).
Kurz, B., Jost, B. & Schunke, M. Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarthrit is Cartilage 10, 119–126 (2002).
Csaki, C., Keshishzadeh, N., Fischer, K. & Shakibaei, M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. 75, 677–687 (2008).
Grishko, V. et al. Effects of hyaluronic acid on mitochondrial function and mitochondria-driven apoptosis following oxidative stress in human chondrocytes. J. Biol. Chem. 284, 9132–9139 (2009).
Coon, H. G. The genetics of the mitochondrial DNA of mammalian somatic cells, their hybrids and cybrids. Natl Cancer Inst. Monogr. 48, 45–55 (1978).
Trifunovic, A. Mitochondrial DNA and aging. Biochem. Biophys. Acta 1757, 611–617 (2006).
Acknowledgements
This work was supported by grants from Secretaria I+D+I Xunta Galicia (PGIDIT06PXIC916175PN); Fundación Española de Reumatologia (programa GEN-SER); Instituto de Salud Carlos III (CIBER- CB06/01/0040); Fondo Investigacion Sanitaria-(PI 08/2028); Ministerio Ciencia e Innovacion PLE2009-0144, with participation of FEDER (European Community). Ignacio Rego was supported by Contrato de Apoyo a la Investigación-Fondo Investigación Sanitaria (CA06/01102). Cristina Ruiz-Romero was supported by Programa Miguel Servet, Fondo Investigación Sanitaria-Spain CP09/00114. The authors express their appreciation to Dr Joaquin Arenas and Miguel A. Martin for scientific criticism of their work on the mitochondrion and for their constant support in developing mitochondrial methodologies.
Author information
Authors and Affiliations
Contributions
I. Rego and C. Ruiz-Romero researched data for the article. All authors made substantial contributions to discussion of the content. F. J. Blanco wrote the article. All authors performed review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Blanco, F., Rego, I. & Ruiz-Romero, C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol 7, 161–169 (2011). https://doi.org/10.1038/nrrheum.2010.213
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2010.213
This article is cited by
-
TfR1 mediated iron metabolism dysfunction as a potential therapeutic target for osteoarthritis
Arthritis Research & Therapy (2024)
-
Osteoarthritis: a narrative review of molecular approaches to disease management
Arthritis Research & Therapy (2023)
-
l-carnitine alleviates synovitis in knee osteoarthritis by regulating lipid accumulation and mitochondrial function through the AMPK-ACC-CPT1 signaling pathway
Journal of Orthopaedic Surgery and Research (2023)
-
Study of exopolysaccharide produced by Streptomyces rochie strain OF1 and its effect as ameliorative on osteoarthritis in rats via inhibiting TNF-α/COX2 pathway
Journal of Genetic Engineering and Biotechnology (2023)
-
Conversion of senescent cartilage into a pro-chondrogenic microenvironment with antibody-functionalized copper sulfate nanoparticles for efficient osteoarthritis therapy
Journal of Nanobiotechnology (2023)