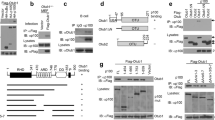
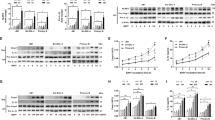
Abstract
NF-κB is usually activated by signal-induced, ubiquitin-mediated degradation of its inhibitor, IκB. This process is initiated by phosphorylation of IκB by the IκB kinase (IKK) complex, predominantly by the IKKβ catalytic subunit, and requires the regulatory subunit IKKγ (NEMO). Another activation pathway, with no known physiological inducers, involves ubiquitin-mediated processing of the NF-κB2 inhibitory protein p100 and is dependent on phosphorylation of p100 by IKKα. We show here that B cell–activating factor (BAFF) activates this second pathway and that this requires the BAFF receptor (BAFF-R), the NF-κB–inducing kinase (NIK) and protein synthesis, but not NEMO. This NEMO-independent cascade is physiologically relevant for the survival and, hence, progression of maturing splenic B cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ghosh, S. & Karin, M. Missing pieces in the NF-κB puzzle Cell 109 (Suppl) 81–96 (2002).
Caamano, J. & Hunter C.A. NF-κB family of transcription factors: central regulators of innate and adaptive immune functions Clin. Microbiol. Rev. 15, 414–429 (2002).
Franzoso, G. et al. Mice deficient in nuclear factor (NF)-κB/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture J. Exp. Med. 187, 147–159 (1998).
Caamano, J.H. et al. Nuclear factor (NF)-κB2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses J. Exp. Med. 187, 185–196 (1998).
Gugasyan, R. et al. Rel/NF-κB transcription factors: key mediators of B-cell activation Immunol. Rev. 176, 134–140 (2000).
Franzoso, G. et al. Requirement for NF-κB in osteoclast and B cell differentiation Genes Dev. 11, 3482–3496 (1997).
Iotsova, V. et al. Osteopetrosis in mice lacking NF-κB1 and NF-κB2 Nature Med. 3, 1285–1289 (1997).
Mackay, F. & Mackay, C.R. The role of BAFF in B-cell maturation, T-cell activation and autoimmunity. Trends Immunol. 23, 113–115 (2002).
Rolink, A.G. & Melchers, F. BAFFled B cells survive and thrive: roles of BAFF in B-cell development. Curr. Opin. Immunol. 14, 266–275 (2002).
Do, R.K. & Chen-Kiang S. Mechanism of BLyS action in B cell immunity. Cytokine Growth Factor Rev. 13, 19–25 (2002).
Mackay, F. & Browning, J.L. BAFF: A fundamental survival factor for B cells. Nature Rev. Immunol. 2, 465–475 (2002).
Thompson, J.S. et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science 293, 2108–2111 (2001).
Khare, S.D. et al. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc. Natl. Acad. Sci. USA 97, 3370–3375 (2000).
Dorner, T. & Putterman C. B cells, BAFF/zTNF4, TACI, and systemic lupus erythematosus. Arthritis Res. 3, 197–199 (2001).
Groom, J. et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren's syndrome. J. Clin. Invest. 109, 59–68 (2002).
Schiemann, B. et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 293, 2111–2114 (2001).
Loder, F. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J. Exp. Med. 190, 75–89 (1999).
Khan, W.N. Regulation of B lymphocyte development and activation by Bruton's tyrosine kinase. Immunol. Res. 23, 147–156 (2001).
Grossmann, M. et al. The anti-apoptotic activities of Rel and RelA required during B-cell maturation involve the regulation of Bcl–2 expression. EMBO J. 19, 6351–6360 (2000).
Rolink, A.G. et al. Precursor B cell receptor-dependent B cell proliferation and differentiation does not require the bone marrow or fetal liver environment. J. Exp. Med. 191, 23–32 (2000).
Su, T.T. & Rawlings, D.J. Transitional B lymphocyte subsets operate as distinct checkpoints in murine splenic B cell development. J. Immunol. 168, 2101–2110 (2002).
Cariappa, A. et al. Nuclear factor κB is required for the development of marginal zone B lymphocytes. J. Exp. Med. 192, 1175–1182 (2000).
Kanno, T. et al. Human T-cell leukemia virus type I Tax-protein-mediated activation of NF-κB from p100 (NF-κB2)-inhibited cytoplasmic reservoirs. Proc. Natl. Acad. Sci. USA 91, 12634–12638 (1994).
Xiao, G. et al. NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol. Cell 7, 401–409 (2001).
Senftleben, U. et al. Activation by IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science 293, 1495–1499 (2001).
Yamada, T. et al. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-κB-inducing kinase. J. Immunol. 165, 804–812 (2000).
Kaisho, T. et al. IκB kinase α is essential for mature B cell development and function. J. Exp. Med. 193, 417–426 (2001).
Do, R.K. et al. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J. Exp. Med. 192, 953–964 (2000).
Solan, N.J. et al. RelB cellular regulation and transcriptional activity are regulated by p100. J. Biol. Chem. 277, 1405–1418 (2002).
Yamaoka, S. et al. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell 93, 1231–1240 (1998).
Batten, M. et al. BAFF mediates survival of peripheral immature B lymphocytes. J. Exp. Med. 192, 1453–1465 (2000).
Ellinger-Ziegelbauer, H. et al. Direct activation of the stress-activated protein kinase (SAPK) and extracellular signal-regulated protein kinase (ERK) pathways by an inducible mitogen-activated protein Kinase/ERK kinase kinase 3 (MEKK) derivative. J. Biol. Chem. 272, 2668–2674 (1997).
Dignam, J.D. et al. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11, 1475–1489 (1983).
Krappmann, D. et al. Different mechanisms control signal-induced degradation and basal turnover of the NF-κB inhibitor IκB α in vivo. EMBO J. 15, 6716–6726 (1996).
Acknowledgements
We thank C. Sibley and C. Scheidereit for the 70Z/3 and 1.3E2 cells, K. Kelly for critical reading of the manuscript and A. Fauci for continued support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Claudio, E., Brown, K., Park, S. et al. BAFF-induced NEMO-independent processing of NF-κB2 in maturing B cells. Nat Immunol 3, 958–965 (2002). https://doi.org/10.1038/ni842
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni842
This article is cited by
-
NF-κB-Inducing Kinase Provokes Insulin Resistance in Skeletal Muscle of Obese Mice
Inflammation (2023)
-
RelB upregulates PD-L1 and exacerbates prostate cancer immune evasion
Journal of Experimental & Clinical Cancer Research (2022)
-
Advancing Biologic Therapy for Refractory Autoimmune Hepatitis
Digestive Diseases and Sciences (2022)
-
Deletion of IKK2 in haematopoietic cells of adult mice leads to elevated interleukin-6, neutrophilia and fatal gastrointestinal inflammation
Cell Death & Disease (2021)
-
The activation of BAFF/APRIL system in spleen and lymph nodes of Plasmodium falciparum infected patients
Scientific Reports (2020)