Abstract
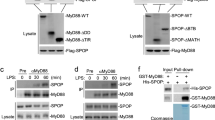
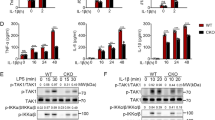
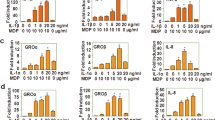
Integrins are critical for the migration and function of leukocytes in inflammation. However, the interaction between integrin αM (CD11b), which has high expression in monocytes and macrophages, and Toll-like receptor (TLR)-triggered innate immunity remains unclear. Here we report that CD11b deficiency enhanced TLR-mediated responses in macrophages, rendering mice more susceptible to endotoxin shock and Escherichia coli–caused sepsis. CD11b was activated by TLR-triggered phosphatidylinositol 3-OH kinase (PI(3)K) and the effector RapL and fed back to inhibit TLR signaling by activating the tyrosine kinases Src and Syk. Syk interacted with and induced tyrosine phosphorylation of MyD88 and TRIF, which led to degradation of these adaptor molecules by the E3 ubiquitin ligase Cbl-b. Thus, TLR-triggered, active CD11b integrin engages in crosstalk with the MyD88 and TRIF pathways and subsequently inhibits TLR signaling in innate immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
Accessions
GenBank/EMBL/DDBJ
NCBI Reference Sequence
References
Barton, G.M. & Medzhitov, R. Toll-like receptor signaling pathways. Science 300, 1524–1525 (2003).
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Liew, F.Y., Xu, D., Brint, E.K. & O'Neill, L.A. Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5, 446–458 (2005).
Marshak-Rothstein, A. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6, 823–835 (2006).
O'Neill, L.A. When signaling pathways collide: positive and negative regulation of Toll-like receptor signal transduction. Immunity 29, 12–20 (2008).
Liu, Y., Penninger, J. & Karin, M. Immunity by ubiquitylation: a reversible process of modification. Nat. Rev. Immunol. 5, 941–952 (2005).
O'Neill, L.A. & Bowie, A.G. The family of five: TIR-domain containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7, 353–364 (2007).
Abram, C.L. & Lowell, C.A. The expanding role for ITAM-based signaling pathways in immune cells. Sci. STKE 2007, re2 (2007).
Ivashkiv, L. Cross-regulation of signaling by ITAM-associated receptors. Nat. Immunol. 10, 340–347 (2009).
Bouchon, A., Facchetti, F., Weigand, M.A. & Colonna, M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410, 1103–1107 (2001).
Hamerman, J.A., Tchao, N.K., Lowell, C.A. & Lanier, L.L. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat. Immunol. 6, 579–586 (2005).
Ivashkiv, L. A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat. Immunol. 8, 816–822 (2008).
Abram, C.L. & Lowell, C.A. The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 27, 339–362 (2009).
Kinashi, T. Intercellular signalling controlling integrin activation in lymphocytes. Nat. Rev. Immunol. 5, 546–559 (2005).
Evans, R. et al. Integrins in immunity. J. Cell Sci. 122, 215–225 (2009).
Mócsai, A. et al. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat. Immunol. 7, 1326–1333 (2006).
Lefort, C.T. et al. Outside-in signal transmission by conformational changes in integrin Mac-1. J. Immunol. 186, 6460–6468 (2009).
Abdelbaqi, M. et al. Regulation of dextran sodium sulfate induced colitis by leukocyte β2 integrins. Lab. Invest. 86, 380–390 (2006).
Ehirchiou, D. et al. CD11b facilitates the development of peripheral tolerance by suppressing Th17 differentiation. J. Exp. Med. 204, 1519–1524 (2007).
Hammerberg, C., Katiyar, S.K., Carroll, M.C. & Cooper, K.D. Activated complement component 3 (C3) is required for ultraviolet induction of immunosuppression and antigenic tolerance. J. Exp. Med. 187, 1133–1138 (1998).
Zhang, M. et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat. Immunol. 5, 1124–1133 (2004).
Tang, H. et al. Endothelial stroma programs hematopoietic stem cells to differentiate into regulatory dendritic cells through IL-10. Blood 108, 1189–1197 (2006).
Xia, S. et al. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells, maintaining liver tolerance. Blood 112, 3175–3185 (2008).
Li, Q., Guo, Z., Xu, X., Xia, S. & Cao, X. Pulmonary stromal cells induce the generation of regulatory DC attenuating T-cell-mediated lung inflammation. Eur. J. Immunol. 38, 2751–2761 (2008).
Perera, P. et al. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J. Immunol. 166, 574–581 (2001).
Behrens, E. et al. Complement receptor 3 ligation of dendritic cells suppresses their stimulatory capacity. J. Immunol. 178, 6268–6279 (2007).
Haziot, A. et al. Resistance to endotoxin shock and reduced dissemination of Gram-negative bacteria in CD14-deficient mice. Immunity 4, 407–414 (1996).
Gao, L. & Kwaik, Y.A. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 8, 306–313 (2000).
Hirahashi, J. et al. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity 25, 271–283 (2006).
Ashley, M. et al. Suppressor of cytokine signaling negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat. Immunol. 7, 148–155 (2006).
Bachmaier, K. et al. E3 ubiquitin ligase Cbl-b regulates the acute inflammatory response underlying lung injury. Nat. Med. 13, 920–926 (2007).
Nikolaj, B., Steen, G. & Søren, B. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294, 1351–1362 (1999).
Shimaoka, M., Takagi, J. & Springer, T.A. Comformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 31, 485–516 (2002).
Susannah, E. et al. Differential activation of LFA-1 and Mac-1 ligand binding domains. Biochem. Biophys. Res. Commun. 337, 142–148 (2005).
Wang, L. et al. Indirect inhibition of Toll-like receptor and type I interferon responses by ITAM-coupled receptors and integrins. Immunity 32, 518–530 (2010).
Adams, R.A., Davalos, C.S., Tsigelny, I. & Akassoglou, K. Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: lessons from multiple sclerosis. Curr. Med. Chem. 14, 2925–2936 (2007).
Hu, X., Wohler, J.E., Dugger, K.J. & Barnum, S.R. β2-integrins in demyelinating disease: not adhering to the paradigm. J. Leukoc. Biol. 87, 397–403 (2010).
Tatsukata, K. et al. TANK is a negative regulator of Toll-like receptor signaling and is critical for the prevention of autoimmune nephritis. Nat. Immunol. 10, 965–973 (2009).
Silver, K.L. et al. MyD88-dependent autoimmune disease in Lyn-deficient mice. Eur. J. Immunol. 37, 2734–2743 (2007).
Zhang, X., Majlessi, L., Deriaud, E., Leclerc, C. & Lo-man, R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity 31, 761–771 (2009).
Fang, D. & Liu, Y. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat. Immunol. 29, 870–875 (2001).
Fukao, T. & Koyasu, S. PI3K and negative regulation of TLR signaling. Trends Immunol. 24, 358–363 (2003).
Wang, C. et al. The E3 ubiquitin ligase Nrdp1 'preferentially' promotes TLR-mediated production of type I interferon. Nat. Immunol. 10, 744–752 (2009).
An, H. et al. Phosphatase SHP-1 promotes TLR- and RIG-I-activated production of type I interferon by inhibiting the kinase IRAK1. Nat. Immunol. 9, 542–550 (2008).
Zhang, T. et al. Fibronectin maintains survival of mouse natural killer (NK) cells via CD11b/Src/β-catenin pathway. Blood 114, 4081–4088 (2009).
Acknowledgements
We thank H. An, M. Zhang, T. Zhang, J. Hou, X. Liu and T. Chen for discussions; F. He for statistical assistance; Y. Li and M. Jin for technical assistance; C. Ni for pathological analysis; S. Akira (Research Institute for Microbial Diseases, Osaka University) for Myd88-deficient mice; H. Yao (Zhejiang University) for E. coli; H. Shen (University of Pennsylvania School of Medicine) for L. monocytogenes; and M. Liu (Shanghai Institute of Biochemistry and Cell Biology) for lentiviral vector. Supported by the National Natural Science Foundation of China (30721091 and 30572121), the National Key Basic Research Program of China (2007CB512403 and 2010CB911903) and the Shanghai Committee of Science and Technology.
Author information
Authors and Affiliations
Contributions
X.C. and C.H. designed the experiments; C.H., J.J., S.X., H.L. and N.L. did the experiments; and X.C. and C.H. analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 (PDF 934 kb)
Rights and permissions
About this article
Cite this article
Han, C., Jin, J., Xu, S. et al. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol 11, 734–742 (2010). https://doi.org/10.1038/ni.1908
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1908
This article is cited by
-
Is cell regeneration and infiltration a double edged sword for porcine aortic valve deterioration? A large cohort of histopathological analysis
BMC Cardiovascular Disorders (2022)
-
Vacuolin-1 enhances RA-induced differentiation of human myeloblastic leukemia cells: evidence for involvement of a CD11b/FAK/LYN/SLP-76 axis subject to endosomal regulation that drives late differentiation steps
Cell & Bioscience (2022)
-
The RING finger protein family in health and disease
Signal Transduction and Targeted Therapy (2022)
-
CUL4B negatively regulates Toll-like receptor-triggered proinflammatory responses by repressing Pten transcription
Cellular & Molecular Immunology (2021)
-
Clinical features and expression of type I interferon-inducible genes in systemic lupus erythematosus patients harboring rs1143679 polymorphism in China: a single-center, retrospective study
Clinical Rheumatology (2021)