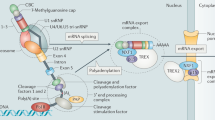
Abstract
The role of the 3′ untranslated region in posttranscriptional regulation of mRNA expression is being elucidated. Here we describe diseases arising from anomalies in this region, that affect the expression of one or more genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Stebbins-Boaz, B. & Richter, J.D. Translational control during early development. Crit. Rev. Eukaryot. Gene Expr. 7, 73–94 (1997).
Gao, F.B. Messenger RNAs in dendrites: localization, stability, and implications for neuronal function. Bioessays 20, 70–8 (1998).
Richter, J.D. Cytoplasmic polyadenylation in development and beyond. Microbiol. Mol. Biol. Rev. 63, 446–56 (1999).
Stutz, A. et al. In vivo antisense oligodeoxynucleotide mapping reveals masked regulatory elements in an mRNA dormant in mouse oocytes. Mol. Cell. Biol. 17, 1759–1767 (1997).
Stutz, A. et al. Masking, unmasking, and regulated polyadenylation cooperate in the translational control of a dormant mRNA in mouse oocytes. Genes Dev. 12, 2535–2548 (1998).
Korade-Mirnics, Z., Babitzke, P. & Hoffman, E. Myotonic dystrophy: molecular windows on a complex etiology. Nucleic Acids Res. 26, 1363–1368 (1998).
Timchenko, L.T. Myotonic dystrophy: the role of RNA CUG triplet repeats. Am. J. Hum. Genet. 64, 360–364 (1999).
Groenen, P.J. et al. Constitutive and regulated modes of splicing produce six major myotonic dystrophy protein kinase (DMPK) isoforms with distinct properties. Hum. Mol. Genet. 9, 605–616 (2000).
Storbeck, C.J., Sabourin, L.A., Waring, J.D. & Korneluk, R.G. Definition of regulatory sequence elements in the promoter region and the first intron of the myotonic dystrophy protein kinase gene. J. Biol. Chem. 273, 9139–9147 (1998).
Strong, P.N. & Brewster, B.S. Myotonic dystrophy: molecular and cellular consequences of expanded DNA repeats are elusive. J. Inherit. Metab. Dis. 20, 159–170 (1997).
Reddy, S. et al. Mice lacking the myotonic dystrophy protein kinase develop a late onset progressive myopathy. Nature Genet. 13, 325–335 (1996).
Davis, B.M., McCurrach, M.E., Taneja, K.L., Singer, R.H. & Housman, D.E. Expansion of a CUG trinucleotide repeat in the 3′ untranslated region of myotonic dystrophy protein kinase transcripts results in nuclear retention of transcripts. Proc. Natl. Acad. Sci. USA 94, 7388–7393 (1997).
Lu, X., Timchenko, N.A. & Timchenko, L.T. Cardiac elav-type RNA-binding protein (ETR-3) binds to RNA CUG repeats expanded in myotonic dystrophy. Hum. Mol. Genet. 8, 53–60 (1999).
Roberts, R. et al. Altered phosphorylation and intracellular distribution of a (CUG)n triplet repeat RNA-binding protein in patients with myotonic dystrophy and in myotonin protein kinase knockout mice. Proc. Natl. Acad. Sci. USA 94, 13221–13226 (1997).
Taneja, K.L., McCurrach, M., Schalling, M., Housman, D. & Singer, R.H. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J. Cell Biol. 128, 995–1002 (1995).
Philips, A.V., Timchenko, L.T. & Cooper, T.A. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science 280, 737–741 (1998).
Timchenko, N.A., Welm, A.L., Lu, X. & Timchenko, L.T. CUG repeat binding protein (CUGBP1) interacts with the 5′ region of C/EBPβ mRNA and regulates translation of C/EBPβ isoforms. Nucleic Acids Res. 27, 4517–4525 (1999).
Sasagawa, N., Takahashi, N., Suzuki, K. & Ishiura, S. An expanded CTG trinucleotide repeat causes trans RNA interference: a new hypothesis for the pathogenesis of myotonic dystrophy. Biochem. Biophys. Res. Commun. 264, 76–80 (1999).
Boucher, C.A. et al. A novel homeodomain-encoding gene is associated with a large CpG island interrupted by the myotonic dystrophy unstable (CTG)n repeat. Hum. Mol. Genet. 4, 1919–1925 (1995).
Thornton, C.A., Wymer, J.P., Simmons, Z., McClain, C. & Moxley, R.T. 3rd. Expansion of the myotonic dystrophy CTG repeat reduces expression of the flanking DMAHP gene. Nature Genet. 16, 407–409 (1997).
Klesert, T.R., Otten, A.D., Bird, T.D. & Tapscott, S.J. Trinucleotide repeat expansion at the myotonic dystrophy locus reduces expression of DMAHP. Nature Genet. 16, 402–406 (1997).
Eriksson, M., Ansved, T., Edstrom, L., Anvret, M. & Carey, N. Simultaneous analysis of expression of the three myotonic dystrophy locus genes in adult skeletal muscle samples: the CTG expansion correlates inversely with DMPK and 59 expression levels, but notDMAHP levels. Hum. Mol. Genet. 8, 1053–1060 (1999).
Chen, C.Y. & Shyu, A.B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem. Sci. 20, 465–470 (1995).
Peng, S.S., Chen, C.Y., Xu, N. & Shyu, A.B. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 17, 3461–3470 (1998).
Rimokh, R. et al. Rearrangement of CCND1 (BCL1/PRAD1) 3′ untranslated region in mantle- cell lymphomas and t(11q13)-associated leukemias. Blood 83, 3689–3696 (1994).
Chagnovich, D., Fayos, B.E. & Cohn, S.L. Differential activity of ELAV-like RNA-binding proteins in human neuroblastoma. J. Biol. Chem. 271, 33587–33591 (1996).
Chagnovich, D. & Cohn, S.L. Binding of a 40-kDa protein to the N-myc 3′-untranslated region correlates with enhanced N-myc expression in human neuroblastoma. J. Biol. Chem. 271, 33580–33586 (1996).
Chagnovich, D. & Cohn, S.L. Activity of a 40 kDa RNA-binding protein correlates with MYCN and c-fos mRNA stability in human neuroblastoma. Eur. J. Cancer 33, 2064–2067 (1997).
Lai, W.S. et al. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol. Cell. Biol. 19, 4311–4323 (1999).
Carballo, E., Lai, W.S. & Blackshear, P.J. Feedback inhibition of macrophage tumor necrosis factor-α production by tristetraprolin. Science 281, 1001–1005 (1998).
Carballo, E., Lai, W.S. & Blackshear, P.J. Evidence that tristetraprolin is a physiological regulator of granulocyte-macrophage colony-stimulating factor messenger RNA deadenylation and stability. Blood 95, 1891–1899 (2000).
Kontoyiannis, D., Pasparakis, M., Pizarro, T.T., Cominelli, F. & Kollias, G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10, 387–398 (1999).
Weiss, I.M. & Liebhaber, S.A. Erythroid cell-specific mRNA stability elements in the α2-globin 3′ nontranslated region. Mol. Cell. Biol. 15, 2457–2465 (1995).
Wang, X., Kiledjian, M., Weiss, I.M. & Liebhaber, S.A. Detection and characterization of a 3′ untranslated region ribonucleoprotein complex associated with human alpha-globin mRNA stability. Mol Cell Biol 15, 1769–1777 (1995); erratum: 15, 2331 (1995).
Morales, J., Russell, J.E. & Liebhaber, S.A. Destabilization of human alpha-globin mRNA by translation anti- termination is controlled during erythroid differentiation and is paralleled by phased shortening of the poly(A) tail. J. Biol. Chem. 272, 6607–6613 (1997).
Russell, J.E. & Liebhaber, S.A. The stability of human beta-globin mRNA is dependent on structural determinants positioned within its 3′ untranslated region. Blood 87, 5314–5323 (1996).
Kobayashi, K. et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature 394, 388–392 (1998).
Fu, L., Minden, M.D. & Benchimol, S. Translational regulation of human p53 gene expression. EMBO J. 15, 4392–4401 (1996).
Acknowledgements
The authors thank S. Antonarakis, F. Negro and J. Schwaller for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Conne, B., Stutz, A. & Vassalli, JD. The 3′ untranslated region of messenger RNA: A molecular ‘hotspot’ for pathology?. Nat Med 6, 637–641 (2000). https://doi.org/10.1038/76211
Issue Date:
DOI: https://doi.org/10.1038/76211
This article is cited by
-
RNA modification in mRNA cancer vaccines
Clinical and Experimental Medicine (2023)
-
The Association of Aquaporin-1 Gene with Marathon Running Performance Level: a Confirmatory Study Conducted in Male Hispanic Marathon Runners
Sports Medicine - Open (2020)
-
Association Between aquaporin-1 and Endurance Performance: A Systematic Review
Sports Medicine - Open (2019)
-
Versican is crucial for the initiation of cardiovascular lumen development in medaka (Oryzias latipes)
Scientific Reports (2019)
-
Regnase-1-mediated post-transcriptional regulation is essential for hematopoietic stem and progenitor cell homeostasis
Nature Communications (2019)