Abstract
Introduction
Epoetin zeta is a recently introduced recombinant erythropoietin, designed to be biologically similar to epoetin alfa. This posthoc analysis evaluated the impact of switching patients with chronic kidney disease (CKD) on hemodialysis from epoetin alfa to epoetin zeta, or vice versa, on hemoglobin concentration, epoetin dose, and patient safety.
Methods
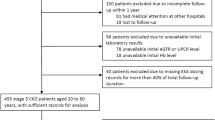
Data were analyzed from three published trials: two 24-week randomized, double-blind (maintenance and induction) studies and a 56-week, open-label, follow-on study involving adult patients with CKD stage 5, maintained on hemodialysis, and receiving epoetin alfa or epoetin zeta. Patients had either completed and switched treatments within the maintenance study, or had completed the induction or maintenance study on epoetin alfa and then switched to, and completed at least 12 weeks of follow-up treatment on, epoetin zeta. Mean hemoglobin levels and epoetin dose were evaluated pre- (0–4 weeks before) and post- (8–12 weeks after) switch, and were considered equivalent for the two treatments if the upper and lower limits of the 95% confidence intervals (CIs) for the intraindividual differences in mean values fell within accepted limits.
Results
Overall, 481 patients were included in the analysis. Mean hemoglobin concentration was maintained at target levels (10.5–12.5 g/dL) throughout the drug switch. The mean differences in hemoglobin concentration and associated 95% CIs following the switch remained within prespecified equivalence limits (±1.0 g/dL). The 95% CIs of the mean difference in weekly epoetin dose postswitch also remained within prespecified equivalence margins (±45 IU/kg; upper limit 17.83 IU/kg, lower limit −10.91 IU/kg). Both treatments were similarly well tolerated.
Conclusion
Our data suggest that epoetin alfa and epoetin zeta therapy can be interchanged without any clinically significant alteration in efficacy, safety, or epoetin dose, in patients with CKD on dialysis receiving stable epoetin maintenance therapy.
Similar content being viewed by others
References
Canadian Erythropoietin Study Group. Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. BMJ. 1990;300:573–578.
Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111:992–1000.
Furuland H, Linde T, Ahlmen J, Christensson A, Strombom U, Danielson BG. A randomized controlled trial of haemoglobin normalization with epoetin alfa in pre-dialysis and dialysis patients. Nephrol Dial Transplant. 2003;18:353–361.
Raftery MJ, Auinger M, Hertlova M. Safety and tolerability of a multidose formulation of epoetin beta in dialysis patients. Collaborative Study Group. Clin Nephrol. 2000;54:240–245.
Ritz E, Eisenhardt A. Early epoetin treatment in patients with renal insufficiency. Nephrol Dial Transplant. 2000;15(Suppl. 3):40–44.
Ross SD, Fahrbach K, Frame D, Scheye R, Connelly JE, Glaspy J. The effect of anemia treatment on selected health-related quality-of-life domains: a systematic review. Clin Ther. 2003;25:1786–1805.
KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47:S11–145.
KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471–530.
National Institute for Health and Clinical Excellence. Clinical Guidelines CG39. Anaemia management in chronic kidney disease. Available at http://guidance.nice.org.uk/CG39. Accessed August 18, 2010.
Locatelli F, Aljama P, Barany P, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004;19(Suppl. 2):ii1–47.
Locatelli F, Covic A, Eckardt KU, Wiecek A, Vanholder R. Anaemia management in patients with chronic kidney disease: a position statement by the Anaemia Working Group of European Renal Best Practice (ERBP). Nephrol Dial Transplant. 2009;24:348–354.
Barbone FP, Johnson DL, Farrell FX, et al. New epoetin molecules and novel therapeutic approaches. Nephrol Dial Transplant. 1999;14(Suppl. 2):80–84.
Elliott S, Pham E, Macdougall IC. Erythropoietins: a common mechanism of action. Exp Hematol. 2008;36:1573–1584.
Jelkmann W. Molecular biology of erythropoietin. Intern Med. 2004;43:649–659.
European Generic Medicines Association. Biosimilar medicines FAQ. Available at http://198.170.119.137/FAQ-biosimilars.htm. Accessed August 18, 2010.
Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008;19:411–419.
Schellekens H. Biosimilar therapeutics - what do we need to consider? NDT Plus. 2009;2:i27–i36.
European Medicines Agency. Retacrit European Public Assessment Report. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000872/human_med_001031.sjsp&murl=menus/medicines/medicines.jsp&jsenabled=true. Accessed August 18, 2010.
European Medicines Agency Committee for Medicinal Products for Human Use (CHMP). Annex to guidelines on similar biological medicinal products containing biotechnologyderived proteins as active substance: non-clinical and clinical issues. Guidance on similar medicinal products containing recombinant erythropoietins. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003921.pdf. Accessed August 18, 2010.
European Medicines Agency Committee for Medicinal Products for Human Use (CHMP). Guidelines on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003920.pdf. Accessed August 18, 2010.
European Medicines Agency Committee for Medicinal Products for Human Use (CHMP). Guidelines on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003953.pdf. Accessed August 18, 2010.
Baldamus C, Krivoshiev S, Wolf-Pflugmann M, Siebert-Weigel M, Koytchev R, Bronn A. Long-term safety and tolerability of epoetin zeta, administered intravenously, for maintenance treatment of renal anemia. Adv Ther. 2008;25:1215–1228.
Krivoshiev S, Todorov VV, Manitius J, Czekalski S, Scigalla P, Koytchev R. Comparison of the therapeutic effects of epoetin zeta and epoetin alpha in the correction of renal anaemia. Curr Med Res Opin. 2008;24:1407–1415.
Wizemann V, Rutkowski B, Baldamus C, Scigalla P, Koytchev R. Comparison of the therapeutic effects of epoetin zeta to epoetin alfa in the maintenance phase of renal anaemia treatment. Curr Med Res Opin. 2008;24:625–637.
Kessler M, Goldsmith D, Schellekens H. Immunogenicity of biopharmaceuticals. Nephrol Dial Transplant. 2006;21(Suppl. 5):v9–12.
Covic A, Cannata-Andia J, Cancarini G, et al. Biosimilars and biopharmaceuticals: what the nephrologists need to know - a position paper by the ERA-EDTA Council. Nephrol Dial Transplant. 2008;23:3731–3737.
Declerck PJ. Biotherapeutics in the era of biosimilars: what really matters is patient safety. Drug Saf. 2007;30:1087–1092.
Kramer I. Pharmacy and pharmacology of biosimilars. J Endocrinol Invest. 2008;31:479–488.
Nowicki M. Basic facts about biosimilars. Kidney Blood Press Res. 2007;30:267–272.
Combe C, Tredree RL, Schellekens H. Biosimilar epoetins: an analysis based on recently implemented European medicines evaluation agency guidelines on comparability of biopharmaceutical proteins. Pharmacotherapy. 2005;25:954–962.
Deechongkit S, Aoki KH, Park SS, Kerwin BA. Biophysical comparability of the same protein from different manufacturers: a case study using Epoetin alfa from Epogen and Eprex. J Pharm Sci. 2006;95:1931–1943.
Park SS, Park J, Ko J, et al. Biochemical assessment of erythropoietin products from Asia versus US Epoetin alfa manufactured by Amgen. J Pharm Sci. 2009;98:1688–1699.
Schellekens H. Biosimilar epoetins: how similar are they? Eur J Hosp Pharm. 2004;3:43–47.
Zuniga L, Calvo B. Regulatory aspects of biosimilars in Europe. Trends Biotechnol. 2009;27:385–387.
European Directorate for the Quality of Medicines. The European Pharmacopoeia. 6th edition. Strasbourg, Paris; July 16, 2007. Available at: http://online6.edqm.eu/ep608/. Accessed August 18, 2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Więcek, A., Ahmed, I., Scigalla, P. et al. Switching epoetin alfa and epoetin zeta in patients with Renal Anemia on Dialysis: Posthoc analysis. Adv Therapy 27, 941–952 (2010). https://doi.org/10.1007/s12325-010-0080-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-010-0080-z