Abstract
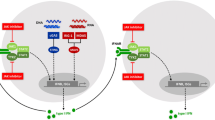
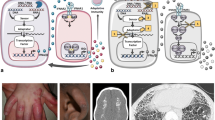
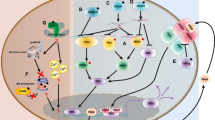
Type I interferons (IFNs) play a central role in the immune defense against viral infections. Type I IFN signaling is activated by pattern recognition receptors upon sensing of viral nucleic acids and induces antiviral programs through modulation of innate and adaptive immune responses. Type I interferonopathies comprise a heterogenous group of genetically determined diseases that are characterized by inappropriate activation of type I IFN. While their phenotypic spectrum is broad, ranging from severe neurological impairment to mild cutaneous disease, systemic autoinflammation, and autoimmunity are commonly shared signs of type I interferonopathies. Although the mechanisms underlying various disease phenotypes associated with inappropriate type I IFN activation have yet to be fully elucidated, our current understanding of the molecular pathogenesis of type I interferonopathies has provided a set of candidate molecules that can be interrogated in search of targeted therapies.

Similar content being viewed by others
Abbreviations
- ADAR:
-
Adenosine deaminase, RNA-specific
- AGS:
-
Aicardi-Goutières syndrome
- CANDLE:
-
Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature syndrome
- cGAMP:
-
Cyclic GMP-AMP
- cGAS:
-
Cyclic GMP-AMP synthase
- IFIH1:
-
Interferon induced with helicase C domain 1
- IFN:
-
Interferon
- IRF:
-
Interferon-regulatory factor
- ISG:
-
Interferon-stimulated gene
- MAVS:
-
Mitochondrial antiviral signaling protein
- MDA5:
-
Melanoma differentiation-associated gene 5
- MYD88:
-
Myeloid differentiation primary-response protein 88
- NF-κB:
-
Nuclear factor-κB
- RIG-I:
-
Retinoic acid-inducible gene 1
- RNASEH2:
-
Ribonuclease H2
- RVCL:
-
Retinal vasculopathy with cerebral leukodystrophy
- SAMHD1:
-
SAM domain and HD domain-containing protein 1
- SAVI:
-
STING-associated vasculopathy, infantile-onset
- SLE:
-
Systemic lupus erythematosus
- STING:
-
Stimulator of interferon genes
- TBK1:
-
TANK-binding kinase 1
- TLR:
-
Toll-like receptor
- TREX1:
-
3′ Repair exonuclease 1
- TRIF:
-
TIR domain-containing adaptor protein inducing IFN-β
References
Stetson DB, Medzhitov R (2006) Type I interferons in host defense. Immunity 25:373–381
Taniguchi T, Takaoka A (2001) A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol 2:378–386
Marshak-Rothstein A (2006) Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol 6:823–835
O’Neill LA, Golenbock D, Bowie AG (2013) The history of Toll-like receptors—redefining innate immunity. Nat Rev Immunol 13:453–460
Atianand MK, Fitzgerald KA (2013) Molecular basis of DNA recognition in the immune system. J Immunol 190:1911–1918
Kawai T, Takahashi K, Sato S et al (2005) IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 6:981–988
Hornung V, Ellegast J, Kim S et al (2006) 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994–997
Goubau D, Schlee M, Deddouche S et al (2014) Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature 514:372–375
Ablasser A, Bauernfeind F, Hartmann G et al (2009) RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol 10:1065–1072
Chiu YH, Macmillan JB, Chen ZJ (2009) RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138:576–591
Sun L, Wu J, Du F et al (2013) Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791
Wu J, Sun L, Chen X et al (2013) Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339:826–830
Xiao TS, Fitzgerald KA (2013) The cGAS-STING pathway for DNA sensing. Mol Cell 51:135–139
Platanias LC (2005) Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 5:375–386
Lovgren T, Eloranta ML, Bave U et al (2004) Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum 50:1861–1872
Napirei M, Karsunky H, Zevnik B et al (2000) Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet 25:177–181
Kawane K, Ohtani M, Miwa K et al (2006) Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 443:998–1002
Crow YJ (2011) Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci 1238:91–98
Aicardi J, Goutieres F (1984) A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol 15:49–54
Lebon P, Badoual J, Ponsot G et al (1988) Intrathecal synthesis of interferon-alpha in infants with progressive familial encephalopathy. J Neurol Sci 84:201–208
Tolmie JL, Shillito P, Hughes-Benzie R et al (1995) The Aicardi-Goutieres syndrome (familial, early onset encephalopathy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis). J Med Genet 32:881–884
Ramantani G, Kohlhase J, Hertzberg C et al (2010) Expanding the phenotypic spectrum of lupus erythematosus in Aicardi-Goutieres syndrome. Arthritis Rheum 62:1469–1477
Rice GI, Forte GM, Szynkiewicz M et al (2013) Assessment of interferon-related biomarkers in Aicardi-Goutieres syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case–control study. Lancet Neurol 12:1159–1169
Vogt J, Agrawal S, Ibrahim Z et al (2013) Striking intrafamilial phenotypic variability in Aicardi-Goutieres syndrome associated with the recurrent Asian founder mutation in RNASEH2C. Am J Med Genet A 161A:338–342
Tüngler V, Schmidt F, Hieronimus S et al (2014) Phenotypic variability in a family with Aicardi-Goutières syndrome due to the common A177T RNASEH2B mutation. Case Rep Clin Med 3:153–156
Crow YJ, Hayward BE, Parmar R et al (2006) Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat Genet 38:917–920
Chowdhury D, Beresford PJ, Zhu P et al (2006) The exonuclease TREX1 is in the SET complex and acts in concert with NM23-H1 to degrade DNA during granzyme A-mediated cell death. Mol Cell 23:133–142
Yang YG, Lindahl T, Barnes DE (2007) Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell 131:873–886
Stetson DB, Ko JS, Heidmann T et al (2008) Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134:587–598
Gall A, Treuting P, Elkon KB et al (2012) Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity 36:120–131
Ablasser A, Hemmerling I, Schmid-Burgk JL et al (2014) TREX1 deficiency triggers cell-autonomous immunity in a cGAS-dependent manner. J Immunol 192:5993–5997
Rice G, Newman WG, Dean J et al (2007) Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi-Goutieres syndrome. Am J Hum Genet 80:811–815
Tungler V, Silver RM, Walkenhorst H et al (2012) Inherited or de novo mutation affecting aspartate 18 of TREX1 results in either familial chilblain lupus or Aicardi-Goutieres syndrome. Br J Dermatol 167:212–214
Crow YJ, Leitch A, Hayward BE et al (2006) Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutieres syndrome and mimic congenital viral brain infection. Nat Genet 38:910–916
Reijns MA, Rabe B, Rigby RE et al (2012) Enzymatic removal of ribonucleotides from DNA is essential for mammalian genome integrity and development. Cell 149:1008–1022
Hiller B, Achleitner M, Glage S et al (2012) Mammalian RNase H2 removes ribonucleotides from DNA to maintain genome integrity. J Exp Med 209:1419–1426
Sparks JL, Chon H, Cerritelli SM et al (2012) RNase H2-initiated ribonucleotide excision repair. Mol Cell 47:980–986
Kim N, Huang SN, Williams JS et al (2011) Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I. Science 332:1561–1564
Kind B, Muster B, Staroske W et al (2014) Altered spatio-temporal dynamics of RNase H2 complex assembly at replication and repair sites in Aicardi-Goutieres syndrome. Hum Mol Genet 23:5950–5960
Gunther C, Kind B, Reijns MA et al (2015) Defective removal of ribonucleotides from DNA promotes systemic autoimmunity. J Clin Invest 125:413–424
Goldstone DC, Ennis-Adeniran V, Hedden JJ et al (2011) HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480:379–382
Hrecka K, Hao C, Gierszewska M et al (2011) Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474:658–661
Laguette N, Sobhian B, Casartelli N et al (2011) SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657
Lahouassa H, Daddacha W, Hofmann H et al (2012) SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol 13:223–228
Goncalves A, Karayel E, Rice GI et al (2012) SAMHD1 is a nucleic-acid binding protein that is mislocalized due to aicardi-goutieres syndrome-associated mutations. Hum Mutat 33:1116–1122
Tungler V, Staroske W, Kind B et al (2013) Single-stranded nucleic acids promote SAMHD1 complex formation. J Mol Med (Berl) 91:759–770
Beloglazova N, Flick R, Tchigvintsev A et al (2013) Nuclease activity of the human SAMHD1 protein implicated in the Aicardi-Goutieres syndrome and HIV-1 restriction. J Biol Chem 288:8101–8110
Ryoo J, Choi J, Oh C et al (2014) The ribonuclease activity of SAMHD1 is required for HIV-1 restriction. Nat Med 20:936–941
Cribier A, Descours B, Valadao AL et al (2013) Phosphorylation of SAMHD1 by cyclin A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep 3:1036–1043
Kretschmer S, Wolf C, Konig N et al (2014) SAMHD1 prevents autoimmunity by maintaining genome stability. Ann Rheum Dis
Rice GI, Kasher PR, Forte GM et al (2012) Mutations in ADAR1 cause Aicardi-Goutieres syndrome associated with a type I interferon signature. Nat Genet 44:1243–1248
Wang Q, Khillan J, Gadue P et al (2000) Requirement of the RNA editing deaminase ADAR1 gene for embryonic erythropoiesis. Science 290:1765–1768
Mannion NM, Greenwood SM, Young R et al (2014) The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell Rep 9:1482–1494
Rice GI, Del Toro DY, Jenkinson EM et al (2014) Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet 46:503–509
Richards A, van den Maagdenberg AM, Jen JC et al (2007) C-terminal truncations in human 3′-5′ DNA exonuclease TREX1 cause autosomal dominant retinal vasculopathy with cerebral leukodystrophy. Nat Genet 39:1068–1070
Schuh E, Ertl-Wagner B, Lohse P et al (2015) Multiple sclerosis-like lesions and type I interferon signature in a patient with RVCL. Neurol Neuroimmunol Neuroinflamm 2:e55
Lee-Kirsch MA, Gong M, Schulz H et al (2006) Familial chilblain lupus, a monogenic form of cutaneous lupus erythematosus, maps to chromosome 3p. Am J Hum Genet 79:731–737
Gunther C, Hillebrand M, Brunk J et al (2013) Systemic involvement in TREX1-associated familial chilblain lupus. J Am Acad Dermatol 69:e179–e181
Lee-Kirsch MA, Chowdhury D, Harvey S et al (2007) A mutation in TREX1 that impairs susceptibility to granzyme A-mediated cell death underlies familial chilblain lupus. J Mol Med 85:531–537
Dale RC, Gornall H, Singh-Grewal D et al (2010) Familial Aicardi-Goutieres syndrome due to SAMHD1 mutations is associated with chronic arthropathy and contractures. Am J Med Genet A 152A:938–942
Liu Y, Jesus AA, Marrero B et al (2014) Activated STING in a vascular and pulmonary syndrome. N Engl J Med 371:507–518
Jeremiah N, Neven B, Gentili M et al (2014) Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest 124:5516–5520
Harley IT, Kaufman KM, Langefeld CD et al (2009) Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat Rev Genet 10:285–290
Baechler EC, Batliwalla FM, Karypis G et al (2003) Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 100:2610–2615
Lee-Kirsch MA, Gong M, Chowdhury D et al (2007) Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat Genet 39:1065–1067
Namjou B, Kothari PH, Kelly JA et al (2011) Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. Genes Immun 12:270–279
Yasutomo K, Horiuchi T, Kagami S et al (2001) Mutation of DNASE1 in people with systemic lupus erythematosus. Nat Genet 28:313–314
Al-Mayouf SM, Sunker A, Abdwani R et al (2011) Loss-of-function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat Genet 43:1186–1188
Manderson AP, Botto M, Walport MJ (2004) The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol 22:431–456
Renella R, Schaefer E, LeMerrer M et al (2006) Spondyloenchondrodysplasia with spasticity, cerebral calcifications, and immune dysregulation: clinical and radiographic delineation of a pleiotropic disorder. Am J Med Genet A 140:541–550
Briggs TA, Rice GI, Daly S et al (2011) Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat Genet 43:127–131
Lausch E, Janecke A, Bros M et al (2011) Genetic deficiency of tartrate-resistant acid phosphatase associated with skeletal dysplasia, cerebral calcifications and autoimmunity. Nat Genet 43:132–137
Gay BB Jr, Kuhn JP (1976) A syndrome of widened medullary cavities of bone, aortic calcification, abnormal dentition, and muscular weakness (the Singleton-Merten syndrome). Radiology 118:389–395
Rutsch F, MacDougall M, Lu C et al (2015) A specific IFIH1 gain-of-function mutation causes Singleton-Merten syndrome. Am J Hum Genet 96:275–282
Jang MA, Kim EK, Now H et al (2015) Mutations in DDX58, which encodes RIG-I, cause atypical Singleton-Merten syndrome. Am J Hum Genet 96:266–274
Bogunovic D, Byun M, Durfee LA et al (2012) Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science 337:1684–1688
Zhang X, Bogunovic D, Payelle-Brogard B et al (2015) Human intracellular ISG15 prevents interferon-alpha/beta over-amplification and auto-inflammation. Nature 517:89–93
Liu Y, Ramot Y, Torrelo A et al (2012) Mutations in proteasome subunit β type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum 64:895–907
Basler M, Kirk CJ, Groettrup M (2013) The immunoproetasome iin antigen processing and other immunolgical functions. Curr Opin Immunol 25:74–80
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinsschaft (Clinical Research Group 249 to M.L.-K. and A.R.) and the Friede Springer Stiftung to M.L.-K.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the Special Issue on The Inflammasome and Autoinflammatory Diseases - Guest Editors: Seth L. Masters, Tilmann Kallinich and Seza Ozen
Rights and permissions
About this article
Cite this article
Lee-Kirsch, M.A., Wolf, C., Kretschmer, S. et al. Type I interferonopathies—an expanding disease spectrum of immunodysregulation. Semin Immunopathol 37, 349–357 (2015). https://doi.org/10.1007/s00281-015-0500-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-015-0500-x