Abstract
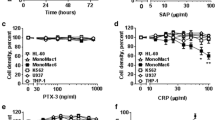
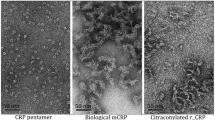
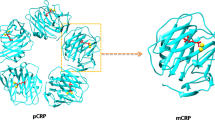
The pentraxins are a family of proteins characterized by cyclic pentameric structure, calcium-dependent ligand binding and sequence homology. The two main representatives of this family are the serum proteins, C-reactive protein (CRP) and serum amyloid P component (SAP). In man CRP is an acute phase reactant which increases up to 1000 fold during the acute phase response whereas SAP is a constitutive protein expressed at about 30 μg/ml. These proteins activate complement through the classical pathway and participate in opsonization of particulate antigens and bacteria. In the past several years it has been determined that both of these pentraxins interact with nuclear antigens including chromatin and small nuclear ribonucleoproteins (snRNPs). Both CRP and SAP have nuclear transport signals which facilitate their entry into the nuclei of intact cells. Furthermore, these pentraxins have been shown to affect the clearance of nuclear antigens in vivo. It is now believed that one of the major functions of the pentraxins could be to interact with the nuclear antigens released from apoptotic or necrotic cells. This interaction could mitigate against deposition of these antigens in tissue and autoimmune reactivity.
Similar content being viewed by others
Abbreviations
- CRP:
-
C-reactive protein
- HSA:
-
human serum albumin
- PC:
-
phosphocholine
- SAP:
-
serum amyloid P component
- snRNP:
-
small nuclear ribonucleoprotein
- SLE:
-
systemic lupus erythematosus
References
Burlingame RW, Rubin RL, Balderas RS & Theofilopoulos AN (1993) J. Clin. Invest. 91: 1687–1696
Tsumita T & Iwanaga M (1963) Nature 198: 1088–1089
Chused TM, Steinberg AD & Talal N (1972) Clin. Exp. Immunol. 12: 465–476
Emlen W & Mannik M (1978) J. Exp. Med. 147: 684–699
Emlen W & Mannik M (1982) J. Exp. Med. 155: 1210–1215
Emlen W & Mannik M (1980) Clin. Exp. Immunol. 40: 264–272
Emlen W & Mannik M (1984) Clin. Exp. Immunol 56: 185–192
Burlingame RW, Boey ML, Starkebaum G & Rubin RL (1994) J. Clin. Invest. 94: 184–192
Mohan C, Adams C, Stanik V & Datta SK (1993) J. Exp. Med. 177: 1367–1381
Osmand AP, Friedenson B, Gewurz H, Painter RH, Hofmann T & Shelton E (1977) Proc. Nat. Acad. Sci. USA 74: 739–743
Gotschlich EC & Edelman EM (1965) Proc. Nat. Acad. Sci. USA 54: 558–566
Volanakis JE & Kaplan MH (1971) Proc. Soc. Exp. Biol. Med. 136: 612–614
Emsley J, White HE, O'Hara BP, Oliva G, Narayanaswamy S, Tickle IJ, Blundell TL, Pepys MB & Wood SP (1994) Nature 367: 338–345
Shrive AK, Cheetham GMT, Holden D, Myles DAA, Turnell QWG, Volanakis JE, Pepys MB, Bloomer AC & Greenhough TJ (1996) Nature Struct. Biol. 3: 346–354
Tillett WS & Francis TJr (1930) J. Exp. Med. 52: 561–571
Salonen E-M, Vartio T, Hedman K & Vaheri A (1984) J. Biol. Chem. 259: 1496–1501
Potempa LA, Siegel J & Gewurz H (1981) J. Immunol. 127: 1509–1514
Nelson SR, Tennent GA, Sethi D, Gower PE, Ballardie FW, Amatayakul-Chantler S & Pepys MB (1991) Clin. Chim. Acta 200: 191–200
Hawkins PN, Lavender JP & Pepys MB (1990) N Engl J Med 323: 508–513
Gitlin JD, Gitlin JI & Gitlin D (1977) Arthritis Rheum. 20: 1491–1499
Robey FA, Jones KD, Tanaka T & Liu T-Y (1984) J. Biol. Chem. 259: 7311–7316
Pepys MB & Butler PJG (1987) Biochem. Biophys. Res. Commun. 148: 308–313
DuClos TW, Zlock LT & Rubin RL (1988) J. Immunol. 141: 4266–4270
Hicks PS, Saunero-Nava L, DuClos TW & Mold C (1992) J. Immunol. 149: 3689–3694
DuClos TW, Mold C & Stump RF (1990) J. Immunol. 145: 3869–3875
Breathnach SM, Kofler H, Sepp N, Ashworth J, Woodrow D, Pepys MB & Hintner H (1989) J. Exp. Med. 170: 1433–1438
DuClos TW, Marnell LL, Zlock LT & Burlingame RW (1991) J. Immunol. 146: 1220–1225
Shephard EG, Smith PJ, Coetzee S, Strachan AF & DeBeer FC (1991) Biochem. J. 279: 257–262
DuClos TW, Zlock L & Marnell LL (1991) J. Biol. Chem. 266: 2167–2171
Minota S, Morino N, Sakurai H, Yamada A & Yazaki Y (1993) Clin. Immunol. Immunopath. 66: 269–271
Saunero-Nava L, Coe JE, Mold C & DuClos TW (1992) Mol. Immunol. 29: 837–845
Robey FA, Jones KD & Steinberg AD (1985) J. Exp. Med. 161: 1344–1356
Shephard GS, VanHelden PD, Strauss M, Böhm L & DeBeer FC (1986) Immunol. 58: 489–494
Butler PJG, Tennent GA & Pepys MB (1990) J. Exp. Med. 172: 13–18
DuClos TW (1989) J. Immunol. 143: 2553–2559
Jewell WS, Marnell LL, Rokeach LA & DuClos TW (1993) Mol. Immunol. 30: 701–708
Pepys MB, Booth SE, Tennent GA, Butler PJG & Williams DG (1994) Clin. Exp. Immunol. 97: 152–157
Theofilopoulos AN & Dixon FJ (1985) Adv. Immunol. 37: 269–391
DuClos TW, Zlock L, Hicks PS & Mold C (1994) Clin. Immunol. Immunopath. 70: 22–27
Burlingame RW, Volzer MA, Harris J & DuClos TW (1996) J. Immunol. 156: 4783–4788
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Du Clos, T.W. The interaction of C-reactive protein and serum amyloid P component with nuclear antigens. Molecular Biology Reports 23, 253–260 (1996). https://doi.org/10.1007/BF00351177
Issue Date:
DOI: https://doi.org/10.1007/BF00351177