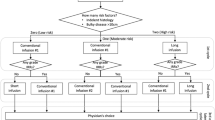
Abstract
Rituximab (Mabthera®) is currently approved for the treatment of multiple subtypes of CD20-expressing, B-cell, non-Hodgkin’s lymphoma. This study aimed to investigate whether rapid infusion of rituximab over 90 min is feasible without compromising patient’s safety, and to reduce resource utilization at a cancer center. This is a prospective and open label study. Lymphoma patients who have received one cycle of rituximab without experiencing grade 3 or 4 infusional reaction were eligible for the rapid infusion of rituximab. Rapid infusion rituximab is infused over 90 min, with 20% of the dose given over the first 30 min and the remaining 80% over 60 min. A total of 79 patients were recruited for this study with a total of 269 infusions administered. Sixty-nine patients (87.3%) received rituximab in combination with chemotherapy. Average number of rituximab infusions administered to patients was 3.4 cycles. Rapid rituximab infusion schedule was well tolerated without any grade 3/4 infusion-related adverse events observed. An average amount of time saved per patient was 10.2 h. Rapid infusion rituximab over 90 min was well tolerated by patients, and shortened infusions have resulted in substantial reduction of resource utilization.
Similar content being viewed by others
References
National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Non-Hodgkin’s Lymphomas. Version 1.2010.
Rituximab (Rituxan). South San Francisco: Genetech Inc. 2008.
Salar A, Casao D, Cervera M, Pedro C, Calafell M, Abella E, et al. Rapid infusion of rituximab with or without steroid-containing chemotherapy: 1-yr experience in a single institution. Eur J Haematol. 2006;77(4):338–40.
Sehn LH, Donaldson J, Filewich A, Fitzgerald C, Gill KK, Runzer N, et al. Rapid infusion rituximab in combination with corticosteroid-containing chemotherapy or as maintenance therapy is well tolerated and can safely be delivered in the community setting. Blood. 2007;109(10):4171–3.
Gibbs S, Pout G, Wimperis J. Rapid infusion rituximab is as effective and safe as conventional infusion regimes in the treatment of diffuse large B cell lymphoma: a 2 year prospective study. Haematologica. 2007;92(Suppl. 2):264.
Ghielmini M, Schmitz SF, Cogliatti SB, Pichert G, Hummerjohann, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly × 4 schedule. Blood. 2004;103:4416–23.
Tuthill M, Crook T, Corbet T, King J, Webb A. Rapid infusion of rituximab over 60 min. Eur J Haematol. 2009;82(4):322–5.
Provencio M, Cerdeira S, Bonilla F, Sanchez A, Espana P. Rapid infusion rituximab in lymphoma treatment. Ann Oncol. 2006;17(6):1027–8.
Siano M, Lerch E, Negretti L, Zucca E, Rodriguez-Abreu D, et al. A phase I-II study to determine the maximum tolerated infusion rate of rituximab with special emphasis on monitoring the effect of rituximab on cardiac function. Clin Cancer Res. 2008;14(23):7935–9.
Acknowledgments
The authors would like to acknowledge the nurses at NCCS Ambulatory Treatment Unit who have assisted us in this study.
Conflict of interest statement
The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Chiang, J., Chan, A., Shih, V. et al. A prospective study to evaluate the feasibility and economic benefits of rapid infusion rituximab at an Asian cancer center. Int J Hematol 91, 826–830 (2010). https://doi.org/10.1007/s12185-010-0583-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-010-0583-z