Article Text
Abstract
Objectives Herein, we investigate the presence and prognostic value of autoantibodies against carbamylated proteins (anti-CarP) in the serum of patients with primary Sjögren's syndrome (pSS).
Patients and methods Serum levels of anti-CarP antibodies were measured in Norwegian patients with pSS (n=78) and corresponding controls (n=74) using ELISA and analysed in relation with exocrine gland function, degree of salivary gland inflammation, signs of ectopic germinal centre (GC) formation and immunological markers. For univariate comparisons, the Mann–Whitney U test and χ2 or Fisher's exact tests were used. Correlations were assessed with Spearman's rank testing. Multivariate regression analyses were used to assess the effect of anti-CarP positivity on clinical manifestations.
Results Of the patients with pSS, 27% were positive for anti-CarP IgG antibodies. Levels of anti-CarP correlated positively with total IgG, IgM, rheumatoid factor and β2-microglobulin. Importantly, after adjusting for confounding factors, patients positive for anti-CarP had significantly higher focus score. Furthermore, positive anti-CarP status coincided with 9.2-fold higher odds of having developed GC-like structures in the minor salivary glands. As a patient group considered having worse disease outcome, individuals with ectopic GC-like structures also presented with significantly higher levels of anti-CarP antibodies.
Conclusions Presence of anti-CarP in patients with pSS is strongly associated with increased focal lymphocytic infiltration, formation of ectopic GC-like structures in minor salivary glands, and diminished salivary gland function. Even taking into consideration our relatively small cohort we believe that anti-CarP antibodies offer new possibilities for identifying patients with more active disease and at risk of developing additional comorbidity.
- Sjøgren's Syndrome
- Autoantibodies
- Inflammation
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Subsequent to translation, nearly all proteins undergo post-translational modifications that affects their function.1 Protein carbamylation is a cyanate-dependent, non-enzymatic conversion of lysine residues and N-terminal amino groups to ε-carbamyl-lysine (homocitrulline) and α-carbamyl amino acids, respectively. Introduction of neutral residues affects the charge distribution within the polypeptide chain. This can result in impairment or loss of a protein's function.2–5 Since urea, a by-product of protein metabolism, and cyanate comprise an equilibrium pair, the level of protein carbamylation is markedly increased in renal insufficiency, leading to chronic uraemia.6 ,7 Interestingly, recent studies demonstrated a novel pathway connecting carbamylation with inflammation via the activation of myeloperoxidase (MPO). MPO is a haem peroxidase released by activated neutrophils. It catalyses the formation of cyanate from thiocyanate in the presence of hydrogen peroxide, leading to homocitrulline formation.8 ,9 This discovery attracted attention to carbamylation in the context of chronic inflammatory and autoimmune diseases.
It is important to remember that antibodies can be a double-edged sword for the host. In addition to their protective effect against pathogens, some individuals produce self-reactive antibodies that contribute to tissue damage in a variety of autoimmune diseases. In the recent years, autoantibodies against post-translationally modified proteins have gained considerable interest in the field of rheumatoid arthritis (RA). The antibodies directed against citrullinated proteins (ACPAs) have become a specific early serological marker of the disease and crucial for patient stratification.10 In addition to citrulline, carbamyl adducts have also been shown to act as neoepitopes in RA11 and juvenile idiopathic arthritis12 resulting in the production of antibodies specifically targeting carbamylated residues (anti-CarP). In an RA cohort and an arthralgia cohort, the presence of anti-CarP correlated with joint destruction and was reported to be predictive of RA development, independent of the presence of anticyclic citrullinated peptide antibodies.13 ,14
Primary Sjögrens's syndrome (pSS) is an autoimmune, chronic inflammatory disease of unknown aetiology. Like most autoimmune diseases, pSS is multifactorial, and genetic predispositions and environmental factors are assumed to be pivotal in disease development. The prevalence of pSS is estimated at approximately 0.09–0.72% of the general population.15 As pSS in characterised by progressive infiltration of mononuclear cells into lacrimal and salivary glands, most patients with pSS suffer from severe symptoms of ocular and oral dryness (keratoconjunctivitis sicca and xerostomia, respectively) and functional impairment of the respective glands.16–19 Severe disease outcomes also include disabling fatigue and development of non-Hodgkin's lymphoma. The prevalence of the latter condition is approximately 16 times more common in patients with pSS as compared with the general population. To date, all therapies thus far tested have been ineffective in reversing the course of pSS.20 ,21
Patients with pSS may present with a variety of autoantibodies. Circulating antinuclear antibodies are present in up to 90% of the patients with pSS, of which antibodies reactive against the ribonucleoprotein antigens Ro/Sjögren's syndrome A antigen (SSA) and La/Sjögren's syndrome B antigen (SSB) are of diagnostic value22 ,23 and may be detectable in the serum several years prior to the diagnosis of pSS.24 In addition, several other autoantibodies have been associated with the disease, including antibodies against the Fc portion of IgG (rheumatoid factor; RF), muscarinic acetylcholine type 3 receptor, carbonic anhydrase, alpha-fodrin, and, to a lesser extent, cyclic citrullinated peptide.25–27 Over the last decade, multiple studies have delineated the importance of autoantibodies as a clinical utility; it remains unknown, however, whether any of the autoantibodies have a direct pathogenic potential or if they merely participate in a secondary response to salivary glands that are already damaged by another process.
In summary, carbamylated proteins are present in inflammatory foci, and there is mounting evidence that post-translational modifications may be pivotal in breaching immune tolerance against self-proteins and driving autoantibody production. This is, to our knowledge, the first study investigating the prevalence of antibodies against carbamylated proteins in patients with pSS. In addition, we assessed whether anti-CarP antibodies may serve as an early biomarker by systematically evaluating their association with clinical characteristics, disease progression and severity.
Materials and methods
Study sample
The Bergen cohort comprises of patients diagnosed with pSS (n=84) who had been consecutively recruited and evaluated at the Department of Rheumatology, Haukeland University Hospital in Bergen, Norway between 2011 and 2015. Patients diagnosed with additional autoimmune diseases or lymphoma (n=6) were excluded; 78 patients were considered eligible for the study. The patients fulfilled the American-European Consensus Group criteria for classification of pSS22 and had their medical history recorded. Sex-matched and approximately age-matched healthy controls (n=74) were recruited from the same geographical area through the Haukeland University Hospital Blood Bank. All patients provided written informed consent.
Detection of anti-CarP antibodies
To investigate the levels of anti-CarP antibodies in serum, a modified ELISA protocol developed by Shi et al11 was used. Fetal calf serum (FCS) was carbamylated by incubation with a final concentration of 1 M potassium cyanate in distilled water for 48 h at 37°C. Following incubation, the potassium cyanate-containing buffer was exchanged with water using Float-a-lyzer G2 (5 kDa) (Spectrum Labs, Breda, the Netherlands). Native and carbamylated FCS (cFCS) was coated on Nunc Maxisorp 96-well plates (Thermo Scientific, Oslo, Norway) overnight at a concentration of 10 μg/mL in 0.1 M carbonate-bicarbonate buffer. Following washing (0.05% Tween in phosphate buffered saline) and blocking (1% bovine serum albumin in phosphate buffered saline), the wells were incubated with serum at a 1:50 dilution in duplicate overnight at 4°C on ice. Bound IgG was detected after incubation with an horseradish peroxidase-conjugated rabbit antihuman IgG antibody (P0214, Dako, Oslo, Norway) for 3.5 h at 4°C on ice. After the last wash, horseradish peroxidase enzyme activity was detected using orthophenylenediamine tablets (Dako). The optical density was measured at 450 nm using an Emax precision microplate reader (Molecular Devices, Sunnyvale, California, USA) and analysed using Soft Max Pro software. To ensure that all results would be comparable, all samples were analysed simultaneously. The background signal of FCS was subtracted from the signal of cFCS in order to analyse the specific anti-CarP reactivity. Samples yielding optical density values more than two SDs above the mean of the anti-CarP measurement of the healthy controls were considered positive for anti-CarP antibodies.
Statistical analysis
The Mann–Whitney U test was used to compare the anti-CarP levels between patients with pSS and the healthy controls. Outliers, identified using the robust regression and outlier removal method (Q=1%), were excluded from the analysis. For univariate comparisons between anti-CarP-positive and anti-CarP-negative patients, the Mann–Whitney U test was used for continuous data and the χ2 or Fisher's exact test, as appropriate, were used for categorical data. Correlations were assessed with Spearman's rank testing. Spearman's partial correlations were modelled to adjust for age, disease duration and total IgG. To evaluate the effect of anti-CarP positivity on clinical manifestations, multivariate Poisson regression analysis was used for analysis of the focus score, and multivariate logistic regression was used for analysis of the presence of germinal centre (GC)-like structures in minor salivary glands. All of the analyses were two-tailed and a p value <0.05 was considered statistically significant. Statistical analyses were performed using Prism V.5 or 6 (GraphPad Software), SPSS statistics V.22 and SAS statistical software V.9.4.
Results
Demographic and clinical features
Serum from patients with pSS (n=78) and healthy controls (n=74) was analysed. Both cohorts were predominantly comprised of women (93.6% of the patients with pSS and 93.2% of controls). The median ages of the patients with pSS and controls were 65 years (range, 31–84) and 56 years (range, 40–70), respectively. At the time of serum collection 18 (23%) were being treated with methotrexate or hydroxychloroquine. Sixteen patients (20.5%) were being treated with corticosteroids. A minor salivary glands biopsy was taken from 68 patients. The focus score was available for 66 patients, and information on the presence of ectopic GC-like structures in the salivary glands was available for 68 patients. Additionally, 66 (84.6%) patients reported extraglandular involvement. All information on the pSS cohort has been summarised in the online supplementary table S1.
Anti-CarP antibody prevalence
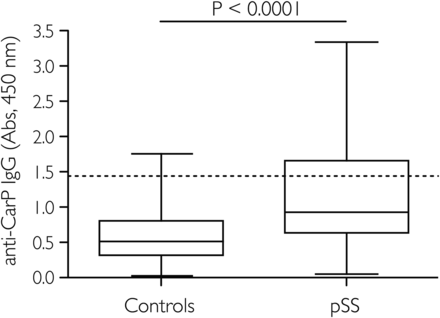
Anti-CarP antibodies were significantly higher in the pSS cohort as compared with the control cohort (26.9% vs 6.8%, respectively; p<0.0001; figure 1). The levels of anti-CarP in the healthy controls are in line with previous reports.13 ,14
Antibodies against carbamylated fetal calf serum (cFCS) in healthy controls and patients with primary Sjögren's syndrome (pSS). The horizontal line in the box-and-whiskers plot indicates the median and the whiskers represent the minimum and maximum values in each group. The dashed line represents the cut-off value for patients considered positive for antibodies against carbamylated proteins, which was calculated as the mean plus two times the SD of the healthy controls. The statistical significance was evaluated by the Mann–Whitney U test. Anti-CarP, autoantibodies against carbamylated proteins.
Associations between clinical manifestations and anti-CarP seropositivity in patients with pSS
Univariate analyses comparing patients with pSS according to their anti-CarP status established that anti-CarP seropositivity was significantly associated with the following measures of pSS disease severity: higher focus scores (p=0.005), presence of GC-like structures (p=0.04) and reduced tear flow (p=0.042). Whereas 71.4% of the anti-CarP-positive patients were positive for RF, the same was true for only 12.3% of the anti-CarP-negative patients. In line with this finding, anti-CarP antibody-positive patients had significantly higher titres of RF as compared with anti-CarP-negative patients (p<0.0001). Furthermore, anti-CarP antibody-positive patients had increased levels of β2-microglobulin (p=0.002), IgG (p=0.011), IgM (p=0.005). By contrast, neither extraglandular involvement (p=1) nor levels of C reactive protein (p=0.4) were significantly associated with presence of anti-CarP antibodies (table 1).
Associations with laboratory parameters in anti-CarP IgG-positive and IgG-negative patients with primary Sjögren's syndrome
We considered also the possibility that the use of therapeutic drugs might have affected the levels of serum markers in our patient cohort.28 However, the use of corticosteroids was very limited and should not have influenced the results. Among the disease-modifying antirheumatic drugs, most of the patients used hydroxychloroquine. We considered the possibility the use of hydroxychloroquines might have influenced the levels of RF, IgM and IgG29–32 and therefore anti-CarP antibody levels. Indeed, our results indicate that hydroxychloroquine may have an effect on the levels of anti-CarP (p=0.016). However, due to the limited number of patients using this drug we believe that a larger study is needed to evaluate if the use of hydroxychloroquine will affect the concentration of circulating anti-CarP IgG.
The variables that were found to be significantly associated with the prevalence of anti-CarP at the univariate level were examined in pairwise correlation analyses. The correlation analyses confirmed that high levels of anti-CarP antibodies are conceding with focus score (r=0.287, p=0.020), low stimulated salivary flow (r=−0.240, p=0.039) and high serum levels of β2-microglobulin (r=0.348, p=0.002), high RF (r=0.443, p<0.0001), total IgM (r=0.318, p=0.005) and total IgG (r=0.320, p=0.004). Importantly, after adjusting for total IgG, age and disease duration, significance was retained for all variables except stimulated salivary flow (table 2). In the context of exocrine gland function, anti-CarP levels did not correlate with reduced tear flow (data not shown).
Correlation between serum levels of anti-CarP IgG and various laboratory parameters in patients with primary Sjögren's syndrome
Multivariable analysis of focus score
To assess the effect of anti-CarP levels on focus score a multivariate Poisson regression model was used. The model adjusted for the potential covariates age and disease duration. In addition, RF, antinuclear antibodies, anti-Ro/SSA and anti-La/SSB were included as confounders since these variables are associated with high focus score.33 ,34 As shown in table 3, anti-CarP positivity predicted increase in the average focus score with 69.4% regardless of the confounders included in the model (p=0.026). It is, however, important to mention that each year of increased disease duration (p=0.001) and anti-La/SSB positivity (p=0.005) also predicted an increase in the average focus score (3.1% and 99.1%, respectively).
Multivariate Poisson regression analysis investigating the effect of anti-CarP IgG positivity on focus score
Association between anti-CarP levels and GC-like structures
Formation of GC-like structures within the salivary gland is emerging as a marker of disease progression and possibly worse clinical outcome. As shown in table 1, frequency of ectopic GC-like structures was 38.9% in anti-CarP seropositive patients with pSS compared with 14.0% in anti-CarP seronegative patients. To further calculate the association between the presence of anti-CarP and GC-like structures we compared the absolute levels of anti-CarP antibodies in patients positive and negative for ectopic GCs. In line with the previous findings, patients presenting GC-like structures in the minor salivary gland had significantly higher levels of circulating anti-CarP antibodies (p=0.027; figure 2). This result led us to investigate a possible effect of anti-CarP positivity on the presence of GC-like structures. After adjusting for age and disease duration, RF, anti-Ro/SSA and anti-La/SSB multivariate logistic regression modelling revealed that patients positive for anti-CarP had 9.2 times higher odds of having developed GC-like structures in the minor salivary glands as compared with anti-CarP negative patients (p=0.019; table 4).
Multivariate logistic regression analysis investigating the effect of anti-CarP IgG positivity on the presence of germinal centre-like structures
Levels of autoantibodies against carbamylated proteins (anti-CarP) antibodies in patients positive or negative for germinal centre (GC)-like structures in the minor salivary glands. The horizontal line indicates the median. The statistical difference was evaluated by the Mann–Whitney U test.
Discussion
Understanding the role of autoantibodies and exploiting their value as biomarkers is of utmost importance for advancing the field of autoimmune diseases and improving patient care. As an example, the identification of ACPA as a specific marker and diagnostic tool for RA has enabled significant understanding of the pathogenesis of this disease due to its strong association with a more severe disease course.35–37 In SS, autoantibodies of different specificities have been associated with various clinical manifestations. The antibodies that are most well studied and integral to diagnosis of patients with SS, anti-Ro/SSA and anti-La/SSB, have been associated with earlier disease onset, rapid disease progression and increased prevalence for developing extraglandular manifestations. Furthermore, the presence of cryoglobulins is strongly correlated with development of lymphoma among patients with pSS.38
In this context, we generally detected anti-CarP antibodies in patients that presented with a more severe disease phenotype. Importantly, this apparent increase in disease activity included several key aspects of SS pathogenesis, ranging from the degree of chronic mononuclear cell inflammation and lymphoid organisation within the salivary glands, to the functional impairment indicated by decreased salivary secretion. Although one may only speculate about the exact mechanism underlying the formation of anti-CarP antibodies, it is now well documented that presence of MPO leads to increased protein carbamylation,39 possibly facilitating a breach in immune tolerance. Concordantly, the activity of circulating MPO is significantly increased in patients with SS.40 Despite this obvious indication and the fact that MPO-dependent carbamylation and antiCarP antibodies has raised considerable interest in the context of inflammatory diseases including atherosclerosis and in particular RA,8 ,13 ,39 ,41 ,42 this is, to our knowledge, the first study investigating the prevalence of antibodies against carbamylated proteins in patients with pSS.
Interestingly, the rate of patients in the pSS cohort that were seropositive for anti-CarP antibodies in this study (27%) corresponds well with the proportion of ACPA-negative patients with RA presenting these antibodies. In line with the previous reports we have not seen any cross-reactivity of anti-CarP antibodies towards citrullinated peptides and/or proteins.11 Only two anti-CarP positive and one anti-CarP negative patients were at the same time ACPA-positive. Recent studies showed presence of circulating antibodies against cFCS years before the development of RA, with a sensitivity ranging between 26% and 57%.11 ,13 ,14 ,43 ,44 We were unable to see any connection between arthritis and the presence of anti-CarP antibodies in our cohort of patients with pSS using Fisher‘s exact test, which indicates that anti-CarP does not obviously associate with secondary arthritis.
Our results also show strong positive correlations between levels of anti-CarP antibodies and total IgG, IgM, RF and β2-microglobulin, indicating that upregulation of anti-CarP occurs in the context of a generalised increase in B cell activity. It is important to mention that 29 (37.2%) of the patients recruited to this study were classified with hypergammaglobulinemia, with elevated levels of at least one of the immunoglobulin isotypes. However, the possibility that the increased levels of anti-CarP antibodies were the sole result of increased IgG levels could be ruled out after adjusting for IgG in a partial correlation analysis. Seropositivity for anti-CarP and RF generally coincided, and the positive pairwise correlation between these two parameters was highly significant. However, this needs to be interpreted with caution due to the high number of patients in whom RF could not be detected (<11 IU/mL).
Indeed, increased mortality in patients with pSS seems strictly related to the increased risk of lymphoproliferative malignancy.45 In this context, it is important to remember that the presence of GC-like structures in the minor salivary glands appears to be a strong predictor for the development of lymphoma46 and is associated with a more severe disease profile.47 ,48 Thus, our finding on an admittedly limited group of patients, that a positive anti-CarP status coincides with more than ninefold higher odds of presenting with GC-like structures suggests need for further studies evaluating anti-CarP as a prognostic biomarker of poor clinical outcome.
To add more weight to our findings we evaluated the presence of anti-CarP IgG antibodies in serum samples from two additional patient cohorts. Cohort 2 (online supplementary figure S1A) represents a patient group recruited in Bergen (consecutive 2004–2009) and cohort 3 comprises samples collected from a Brazilian population (see online supplementary figure S1B). Indeed, the proportion of patients being seropositive for anti-CarP antibodies was relatively stable across these three cohorts. In cohort 2, 13 (28.9%) out of 45 patients with pSS and 18 (19.6%) out of 92 patients from cohort 3 were found positive for anti-CarP IgG antibodies. Corresponding to our findings for cohort 1, also the patients with SS comprised in these additional cohorts had significantly higher levels of anti-CarP antibody compared with healthy controls (p=0.0006 and p=0.016 for cohorts 2 and 3, respectively).
Intriguingly, 40.8% of sera from patients with pSS analysed were positive for IgA anti-CarP antibodies (see online supplementary figure S2) as compared with 26.9% positive for IgG anti-CarP antibodies. Their presence however, had a lower impact on the outcome of pSS (see online supplementary table S2). Although it is premature to draw definite conclusions, one may speculate that anti-CarP IgGs contribute to pathogenesis while IgAs characterise an ‘intermediate’ phase in disease development.
In conclusion, this is the first study delineating the presence and frequency of antibodies against carbamylated proteins in association with relevant disease manifestations of pSS. Anti-CarP antibodies were shown to predict the clinical outcome of pSS. It is reasonable to suggest anti-CarP status as an additional biomarker in the serological profile of patients with pSS because of their close association with degree of focal lymphocytic infiltration of the minor salivary glands, formation of ectopic GC-like structures and diminished salivary flow. New serological markers, allowing early detection, could lead to closer patient follow-up and more timely application of immunosuppressive agents. This would limit irreversible tissue damage and reduce mortality related to the comorbidity.49 ,50 Once validated, quantification of anti-CarP antibodies in the serum could be used to determine disease severity and identifying patients at risk of developing additional comorbidity.
Acknowledgments
All authors met the criteria for authorship. BB, CK and AH measured the levels of anti-CarP using ELISA assay. BB and CK acquired and coordinated data for patients with pSS and healthy controls. CK performed univariate and bivariate statistical analyses. MS modelled the multivariate regression analyses and performed partial correlation. DSH collected and supplied the data of the pSS cohort. MVJ analysed the presence of germinal centre formation. VV, VB, CK, MS, ND, RJ, AH and PM interpreted the data and CK, ND and PM wrote the manuscript. All authors agreed to publish this work and critically reviewed the article. Conception and design of this work was discussed with all the authors.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1 - Online supplement
Footnotes
Handling editor Tore K Kvien
BB, CK and ND contributed equally.
Funding This work was funded by grants from the EC Marie Curie ITN (RAPID no. 290246) and (FP7-HEALTH-F3-2012-306029 ‘TRIGGER’). PM and AH were supported by the grant from the National Science Center (2014/14/E/NZ6/00162, Poland). ND and PM were supported by the Bergen Medical Research Foundation and the Broegelmann Foundation.
Competing interests None declared.
Ethics approval The Regional Committee of Medical and Health Research Ethics (REK 3.2006.3085) and the Ethics Committee of UFES (407.199/2013).
Provenance and peer review Not commissioned; externally peer reviewed.