Article Text
Abstract
Objectives: To evaluate changes in bone mineral density (BMD) in the hands, hip and spine after 1 and 2 years of follow-up, in relation to antirheumatic and antiresorptive therapies and disease and demographic variables in patients with recent-onset rheumatoid arthritis (RA).
Methods: Changes in BMD measured in metacarpals 2–4 by digital x-ray radiogrammetry and in the hip and spine by dual energy x-ray absorptiometry were assessed at baseline and after 1 and 2 years of follow-up in 218 patients with recent-onset RA from the BeSt study, who received one of four treatment strategies: sequential monotherapy (group 1); step-up combination therapy (group 2); initial combination therapy with tapered high-dose prednisone (group 3); or initial combination therapy with infliximab (group 4).
Results: After 1 and 2 years, there was significant BMD loss in all locations, with significantly greater BMD loss in the hands than generalised BMD loss in the hip and spine. Initial combination therapy with prednisone or infliximab were associated with less hand BMD loss compared with initial monotherapy after 1 and 2 years (−0.9 and −1.6%, −0.6 and −1.4%, −1.7 and −3.3%, and −2.6 and −3.6% for group 4–1 after 1 and 2 years, overall p = 0.001 and p = 0.014, respectively).
Progression in erosions was independently associated with increased BMD loss both in the hands and hip after 1 year. The use of bisphosphonates protected only against generalised BMD loss in the hip and spine.
Conclusions: The association between joint damage progression and both hand and generalised BMD loss in RA suggests common pathways between these processes, with hand BMD loss occurring earlier in the disease course than generalised BMD loss.
Statistics from Altmetric.com
Erosions and hand and generalised bone mineral density (BMD) loss in rheumatoid arthritis (RA)1–3 results in functional disability and increased risk of clinical fractures.4–6 Recent studies suggest that pathophysiological mechanisms of focal erosions and hand and generalised BMD loss have common pathways mediated by osteoclasts, in particular by the receptor activator of nuclear factor-κβ ligand.7–9 Clinical studies evaluating BMD in the hands and generalised BMD in the hip and spine of patients with early RA showed associations between high BMD loss and disease severity, as measured by inflammation parameters, (progressive) joint damage and functional disability.10–14
In patients with RA, corticosteroids decrease generalised BMD loss by suppression of inflammatory activity, but as a side-effect, also increase BMD loss.14–19 Treatment with tumour necrosis factor α antagonist (anti-TNFα) might protect against generalised BMD loss.20–22 However, little is known about the effect of corticosteroids23 and anti-TNFα21 on hand BMD loss. While the efficacy of calcium and vitamin D supplements remains inconclusive, use of bisphosphonates has been shown to protect against, especially corticosteroid-induced, generalised BMD loss.24–28 The influence of antiresorptive treatment on hand BMD loss is unclear.29
To investigate the possible common pathological mechanisms of erosions and hand and generalised BMD loss and the effects of different antirheumatic and antiresorptive treatments on BMD loss, we assessed the influence of disease-related factors, antirheumatic treatment strategies and antiresorptive treatments on BMD loss in the hands, hip and spine after 1 and 2 years of follow-up in patients with recent-onset, active RA.
METHODS
Patients and therapy
Details of the BeSt study30 and 1-year changes in generalised BMD loss in the hip and the spine from this cohort14 have been previously reported. This study included 218 of 508 patients from eight investigative centres with analogue hand radiographs and dual energy x-ray absorptiometry (DEXA) measurements of the hip and the lumbar spine at baseline and 1 and 2 years follow-up. Inclusion criteria were diagnosis of RA as defined by the American College of Rheumatology 1987 revised criteria, symptom duration <2 years, age ⩾18 years, and active disease with ⩾6 of 66 swollen joints, ⩾6 of 68 tender joints and either an erythrocyte sedimentation rate of ⩾28 mm/h or a visual analogue scale global health of ⩾20 mm on a scale of 100 mm. Exclusion criteria included previous treatment with disease-modifying anti-rheumatic drugs other than antimalarials and estimated creatinine clearance <75%. Patients were randomised to one of the four treatment strategies: sequential monotherapy (group 1); step-up combination therapy (group 2); initial combination therapy with tapered high-dose prednisone (group 3); or initial combination therapy with infliximab (group 4). Treatment was adjusted using 3-monthly calculations of the disease activity score (DAS, based on a 44 joint count), with patients progressing to the next treatment step in the protocol if DAS >2.4. Calcium supplement (500–1000 mg/day) was recommended to patients with <1000 mg/day calcium intake and vitamin D supplement (cholecalciferol 400 IE/day) to patients with serum vitamin D level below the local reference value. Antiresorptive therapy with oral alendronate (10 mg/day or 70 mg/week) or risedronate (5 mg/day or 35 mg/week) was advised to non-corticosteroid users with a BMD T score ⩽−2.5 SD in the spine and/or hip and to corticosteroids users with a T-score ⩽−1 SD. The ethics committee at each participating centre approved the study protocol and all patients gave written informed consent.
Hand bone mineral density measurements
Standard analogue radiographs of both hands in posteroanterior position, digitalised by a high-resolution 300 DPI scanner (Canon Vidar VXR-12 plus), were used to measure BMD by digital x-ray radiogrammetry (DXR).31 Digital radiographs taken at baseline and/or during the follow-up period were excluded from the analyses due to lack of comparability between the different imaging devices. Mean surrogate hand BMD was calculated from cortical thickness from regions of interest measured at the centre of the second, third and fourth metacarpals through an automated analysis of a standard projection digital radiograph of the hands using the DXR online technology (Sectra, Sweden). Hand BMD measured by DXR seems superior to other BMD measurement devices in detecting inflammation-related bone loss in patients with arthritis.32–34 To avoid biasing dominant and non-dominant hands and to achieve better precision, the mean of both hands was used for the analyses.
Generalised bone mineral density measurements
BMD measurements of the left total hip and the lumbar spine L2–L4 posteroanterior view at baseline and 1 and 2 years follow-up were performed where DEXA was available, using a Hologic 4500 QDR (Hologic, Waltham, Massachusetts, USA) in four centres and a Lunar DPX (Lunar, Madison, Wisconsin, USA) in four centres. All procedures were performed in accordance with the manufacturer’s standardised procedures for hip and spine BMD measurements. Despite differences between the densitometers, the rates of change in BMD, calculated from serial measurements assessed for each patient by the same machine, measurement procedure and references, are comparable.35
Clinical measurements
The following variables were collected at baseline: symptom duration and serum IgM rheumatoid factor (RF); at baseline and 3-monthly: age, body mass index (BMI), C-reactive protein (CRP) levels, the use of calcium and vitamin D supplements, hormone replacement therapy (HRT) and bisphosphonates, and functional ability as measured by the Dutch validated health assessment questionnaire (HAQ); and at baseline and after 1 and 2 years of follow-up: menopausal status, age at menopause, smoking status, alcohol status, previous clinical fractures, osteoporosis in first-degree relatives, estimated daily calcium intake and 25(OH)vitamin D levels. The presence of anti-citrullinated protein antibody was determined from serum samples obtained at baseline or during follow-up. Disease activity was assessed 3-monthly using the DAS, based on the erythrocyte sedimentation rate, the number of swollen joints and the Ritchie articular index for pain in tender joints in a 44 joint count and the visual analogue scale for patient’s global assessment of disease activity (0–100 mm, 0 = best and 100 = worst).36 Radiographic joint damage was assessed using the Sharp–van der Heijde score (SHS), scored after 1 and 2 years of follow-up by two independent physicians blinded for patient-level data and treatment assignment. After 1 year, the intra-observer coefficients were 0.93 and 0.94, and the interobserver coefficient was 0.93. Erosive disease at baseline was defined as erosion score >0.5. Progression of joint damage after 1 year was defined as progression greater than the smallest detectable change (SDC), calculated as 4.18 points in the first year of follow-up.
Statistical analysis
All analyses were performed in an intention-to-treat method using all available data. Changes in BMD were expressed as changes at 1 and 2 years follow-up in absolute BMD values compared with baseline BMD in percentages. Non-parametric tests were performed to compare the median percentages of BMD loss in the hands, hip and spine between the treatment strategies. The p-values derived by these tests were corrected for multiple comparisons by the step-down Bonferroni–Holmes adjustment. Multivariate regression analyses, adjusted for the use of bisphosphonates, vitamin D and calcium supplements, HRT and intra-articular steroids and changes in DAS, HAQ and SHS during follow-up, were used to compare the treatment strategies, independently of differences in antiresorptive treatment and disease activity between the groups. Association among disease-related variables and changes in BMD in the different measurement sites were analysed by univariate regression analysis. Potential independent predictors of BMD loss were evaluated by stepwise multivariate regression analyses performed as forward (conditional) procedures, adjusted for treatment group. All tests were two-tailed and p⩽0.05 was considered statistically significant.
RESULTS
Patient characteristics
In 218 patients BMD measurements in the hands, lumbar spine and total left hip were performed at baseline and 1 and 2 years follow-up. In 27 patients no hand BMD measurements were performed after 1 or 2 years due to logistic reasons and in 20 patients no BMD in the hip or spine measurements after 1 or 2 years were performed due to logistic reasons and in two cases due to bilateral hip prosthesis.
The baseline demographic and disease variables were not significantly different between the four treatment groups (table 1) or with the rest of the BeSt study population (n = 290) (data not shown). The majority of patients were middle-aged, postmenopausal women with recent-onset RA with median symptom duration of 23 weeks. All patients had active disease with a mean (SD) DAS of 4.4 (0.9), 69% of patients had erosive disease at baseline and 58% of patients were anti-citrullinated protein antibody positive at baseline or during follow-up.
Osteoporosis treatment
The use of osteoporosis treatment during the follow-up is summarised in table 2. Of patients advised to take bisphosphonates, only 45% were actually prescribed oral bisphosphonates, of which 66% received alendronate and 34% received risedronate. Thirty-nine per cent of patients with low calcium intake received calcium supplement, and 40% of patients with 25(OH)vitamin D levels below the reference value received vitamin D supplement during the 2 years of follow-up. Forty-five per cent of the patients taking calcium and vitamin D supplements were also taking bisphosphonates. Bisphosphonates were prescribed to significantly more patients in group 3 (initial combination therapy including prednisone) than in the other groups (29% vs 4–9%, overall p<0.0001, and 32% vs 15–17%, overall p = 0.05 during the first year and first 2 years of follow-up, respectively); and more patients in group 3 used calcium supplements (43% vs 19–20%, overall p = 0.005 and 46% vs 22–31%, overall p = 0.039, respectively). There was a trend for increased vitamin D supplements use by patients in group 3 during the first year of follow-up (22% vs 6–15%, overall p = 0.057). More patients received intra-articular steroid injections in groups 1 and 2 than in groups 3 and 4 during the first year of follow-up (overall p = 0.001).
Changes in hand and generalised bone mineral density loss
After 1 year of treatment, the median (IQR) change from baseline in hand BMD was approximately −1.4% (−3.6% to 0.1%; p<0.0001) in the hands compared with −0.9% (−2.9% to 1.7%; p<0.0001) in generalised BMD in the hip and −0.5% (−2.8% to 1.5%; p<0.0001) in the spine. Hand BMD loss was significantly greater than generalised BMD loss after 1 year (hand versus hip p = 0.004, hand versus spine p<0.0001, hip versus spine p = 0.43).
After 2 years of treatment, the median (IQR) change in BMD was approximately −2.5% (−6.0% to −0.2%; p<0.0001) in the hands compared with −0.5% (−2.8% to 2.1%; p<0.0001) in the hip and −1.0% (−3.9% to 1.6%; p<0.0001) in the spine. Hand BMD loss remained significantly greater than generalised BMD loss (hand versus hip and spine p<0.0001, hip versus spine p = 0.46).
Effect of treatment strategies on bone mineral density changes
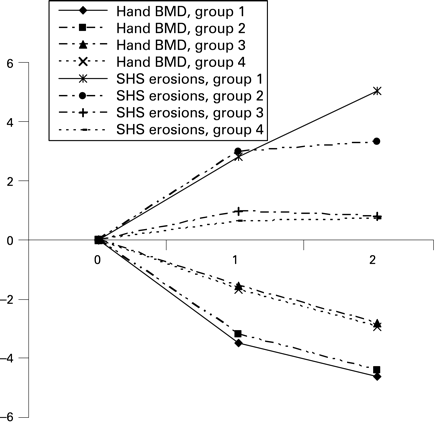
In univariate analyses, patients in the initial monotherapy strategy (group 1) had significantly more BMD loss in the hands after 1 year than patients in the initial combination therapies (groups 3 and 4) (−2.6, −1.7, −0.6 and −0.9% for group 1–4, respectively, overall p = 0.001, group 1 versus 3 p = 0.000, group 1 versus 4 p = 0.021, group 2 versus 3 p = 0.038, group 2 versus 4 p = 0.101, table 3).
Multivariate regression analyses, adjusted for differences in use of antiresorptives between the treatment strategies during follow-up, also showed significant less BMD loss in the hands in the initial combination therapies (data not shown).
The amount of BMD loss in the hands was associated with disease severity (fig 1). Multivariate regression analyses, adjusted for differences in antiresorptives and changes in disease activity (DAS, HAQ and SHS after 1 year) between the groups, showed no significant differences in hand BMD loss between the treatment strategies anymore (data not shown). Differences in hand BMD loss between the treatment groups remained significant after 2 years of treatment in univariate analyses (−3.6, −3.3, −1.4 and −1.6% for groups 1–4, respectively, overall p = 0.014, group 1 vs 3 p = 0.009, group 1 vs 4 p = 0.033, group 2 vs 3 p = 0.204, group 2 vs 4 p = 0.216). However, after correction for disease activity and use of antiresorptives, hand BMD loss was again no longer statistically significant between the treatment groups (data not shown). There were no statistically significant differences between the four treatment groups in generalised BMD loss in the hip (overall p = 0.42 and p = 0.52 after 1 and 2 years of follow-up, respectively) and the spine (overall p = 0.52 and p = 0.93, respectively).14
Given the dynamics of DAS-directed treatment adjustments in all four treatment groups, patients who started treatment with prednisone (group 3) were eligible for discontinuation of that drug after at least 28 weeks (at 2 years, 82% had discontinued prednisone due to a good response or failure to respond), whereas patients who did not respond to previous disease-modifying anti-rheumatic drug therapy in groups 2 (step up to combination therapy) and 4 (initial combination therapy with infliximab) were allowed to begin prednisone starting at 12 and 15 months, respectively. In the patients who used prednisone in all groups, the mean (SD) cumulative dose was 2428 (388) mg and 2796 (1197) mg per patient during the first year and first 2 years of follow-up, respectively. Subanalyses adjusted for differences in disease activity and anti-osteoporotic treatment in multivariate analyses showed no differences in hand or generalised BMD loss after 1 year between patients exposed to and naive to prednisone (data not shown). In the second year of follow-up, patients who did not respond to previous disease-modifying anti-rheumatic drug therapy in groups 1–3 were allowed to receive infliximab. The mean (SD) cumulative dose of infliximab in all groups was 29.5 (8.5) mg/kg and 37.0 (21.9) mg/kg per patient during the first year and the first 2 years of follow-up, respectively. There were no differences in hand or generalised BMD loss after 1 and 2 years between patients exposed to and naive to infliximab (data not shown).
Determinants of bone mineral density loss
Univariate linear regression analyses showed that higher age, postmenopausal status and current smoking status were associated with greater hand BMD loss after 1 year (table 4), but demographic variables were not associated with generalised BMD loss in the hip or spine. Of disease-related variables, progression in erosion scores greater than the SDC was associated with increased BMD loss both in the hands and hip after 1 year of follow-up. Further, higher CRP levels at baseline and a lower decrease in CRP levels after 1 year and higher HAQ scores at baseline were associated with increased BMD loss in the hands and higher erosion scores at baseline with increased BMD loss in the hip. There was a trend of increased hand BMD loss in patients who were positive for RF after 1 year (p = 0.055). The use of bisphosphonates, calcium and vitamin D supplements was associated with reduced generalised BMD loss.
Multivariate regression analyses showed that postmenopausal status was an independent risk factor of BMD loss in the hands (table 5). Increase in erosion score after 1 year was associated with both greater hand and generalised BMD loss in the hip. Higher CRP levels at baseline were independently associated with increased hand BMD loss. The use of bisphosphonates was independently associated with reduced generalised BMD loss in both the hip and spine.
DISCUSSION
In this large longitudinal study we compared changes in hand BMD, measured by DXR, with changes in generalised BMD in the hip and the spine, measured by DEXA, in 218 patients with recent-onset RA after 1 and 2 years of DAS-steered treatment in the BeSt trial. There are several important findings. Hand BMD loss was greater than generalised BMD loss in the hip and spine. Patients treated with initial combination therapy with tapered high-dose corticosteroids or anti-TNFα infliximab had less hand BMD loss due to better suppression of inflammation. Both hand and generalised BMD loss were associated with progression of radiographic destruction. Bisphosphonates protected only against generalised BMD loss.
In Caucasian populations, the rate of BMD loss in the metacarpals has been found to be about −1.2 to −1.5% per year after the menopause.37 In our population, BMD loss was approximately −2.3% after 1 year and −4.4% after 2 years in postmenopausal women, indicating that postmenopausal patients with recent-onset RA may experience a twofold increase in BMD loss in the hands per year, despite aggressive suppression of inflammation by DAS-directed treatment. However, comparisons should be interpreted with caution due to possible demographic differences between populations.
BMD loss in the hands is common in recent-onset RA, whereas generalised BMD loss primarily occurs during a later course of the disease.38 39 Despite significant reduction in disease activity over the treatment period, BMD loss in the hands was on average two to three times more severe than generalised BMD loss in the hip or spine in our patients. The majority of our patients with hand BMD loss had no generalised BMD loss in the hip (72%) and spine (80%) during 2 years of follow-up.
We found significantly more BMD loss after 1 and 2 years in the hands than in the hip and spine. There are several possible explanations for this finding. First, two different techniques were used to measure different components of the bone: DXR estimates cortical BMD loss and DEXA measures both cortical and trabecular BMD loss. This might indicate that the cortical barrier of bone is more exposed to inflammation-induced osteoclasts activation than the trabecular site. However, previous studies studying BMD loss in the hands by DEXA, the gold standard for bone assessment, also reported more severe hand BMD loss compared with generalised BMD loss in patients with RA.40–42 Second, greater changes in BMD in the hands, measured by DXR, than in generalised BMD loss, measured by DEXA, may be due to the higher precision of the DXR technique and the averaging of three bones in one hand and the averaging of both hands.31 As a result, DXR may be more sensitive in tracing changes in BMD loss than DEXA. Third, the process of hand BMD loss may be more sensitive to or more directly influenced by cytokine stimulation originating in adjacent inflamed synovial tissue compared with the process of generalised BMD loss at locations with undetected local inflammation. Fourth, hand BMD loss that is more severe and difficult to suppress may be a reflection of ongoing inflammation that remains undetected by clinical observation. Previously, Brown et al showed synovitis detected with magnetic resonance imaging in patients with RA in clinical remission.43 Lastly, the protective effect of bisphosphonates was only observed against generalised, and not hand, BMD loss. A previous study showed that bisphosphonates were effective against hand BMD loss in patients without RA.29 To our knowledge, this is the first study measuring the effect of antiresorptive treatment on hand BMD loss in patients with recent-onset RA. A possible explanation for the conflicting results is that the inflammation nearby the metacarpals may counteract the antiresorptive effect of bisphosphonates due to high resorptive activity of the osteoclasts. A limitation to our study is that the guidelines for anti-osteoporotic treatment in patients who were osteopenic and osteoporotic were poorly implemented: only 45% of patients requiring bisphosphonates were actually prescribed bisphosphonates during the 2 years of follow-up. However, despite the low prescription, the use of bisphosphonates protected against generalised BMD loss.
We found significantly less hand BMD loss in the initial combination group with high-dose prednisone compared with the initial monotherapy groups. In a previous double-blind study comparing oral prednisolone 7.5 mg/day for 2 years with placebo in patients with early RA, the prednisone group had less hand BMD loss measured by DXR after 1 and 2 years.23 This suggests that the benefits from quick effective suppression of disease activity with corticosteroids exceed the direct negative influence on hand BMD. We did not find differences in changes in BMD in the hip and the spine after 1 and 2 years follow-up between the initial group with prednisone and the other treatment groups.14 17
In our study, patients who received initial combination therapy with infliximab had significantly less hand BMD loss than patients who received conventional therapy in groups 1 and 2 after 1 year, but there were no differences in generalised BMD loss. In a group of 102 patients with RA with a disease duration of 1–49 years, Vis et al showed consistent BMD in the spine and hip but significant BMD loss in the hands (−0.8%) after 1 year of treatment with infliximab.21 However, comparisons should be interpreted with caution due to differences in demographic and RA-related variables between the two populations, such as use of antiresorptives and shorter disease duration in our population, which is associated with more rapid hand BMD loss.44
The independent associations between focal erosions and hand and generalised BMD loss support the current understanding that these three processes share common pathways mediated by the cellular action of osteoclasts.7 45 46 BMD loss involves elevated bone loss in the hands in the early course of the disease and generalised BMD loss often during a later phase of RA.
In conclusion, in patients with recent-onset RA, the suppression of inflammation with effective treatment strategies is essential for bone preservation. The association between progressive erosive disease and high hand and generalised BMD loss indicates common pathophysiological mechanisms, hand BMD loss occurring more often in the early phase of the disease than generalised BMD loss. Identifying therapeutic opportunities to prevent or treat all these forms of bone loss in patients with RA remains a challenge.
Acknowledgments
The authors thank the following participants of the Foundation for Applied Rheumatology Research for their contributions to the design and the conduct of the study (all locations are in The Netherlands): W M de Beus, MD (Medical Center Haaglanden, the Hague); G Collée, MD (Medical Center Haaglanden, The Hague); J A P M Ewals, MD (Haga Hospital, The Hague); A H Gerards, MD (Vlietland Hospital, Schiedam); J B A M Grillet, MD (De Honte Hospital, Terneuzen); K H Han, MD (Medical Center Rijnmond-Zuid); J M W Hazes, MD (Erasmus Medical Center, Rotterdam); H M J Hulsmans, MD (Haga Hospital, The Hague); M H de Jager, MD (Albert Schweitzer Hospital, Dordrecht); J M de Jonge-Bok, MD (Groene Hart Hospital, Gouda); P J S M Kerstens, MD (Jan van Breemen Institute, Amsterdam); M V van Krugten, MD (Walcheren Hospital, Vlissingen); H van der Leeden, MD (retired); M F van Lieshout-Zuidema, MD (Spaarne Hospital, Hoofddorp); A Linssen, MD (Kennemer Gasthuis, Haarlem); P A H M van der Lubbe, MD (Vlietland Hospital, Schiedam); H K Markusse, MD (deceased); A J Peeters, MD (Reinier de Graaf Hospital, Delft); H K Ronday, MD (Haga Hospital, The Hague); D van Schaardenburg, MD (VU Medical Center, Amsterdam and Jan van Breemen Institute, Amsterdam); P E H Seys, MD (Lievensberg Hospital, Bergen op Zoom); R M van Soesbergen, MD (retired); P B J de Sonnaville, MD (Oosterschelde Hospital, Goes); I Speyer, MD (Bronovo Hospital, The Hague); J Ph Terwiel, MD (Spaarne Hospital, Hoofddorp); A E Voskuyl, MD (VU Medical Center, Amsterdam); M L Westedt, MD (Bronovo Hospital, The Hague); S ten Wolde, MD (Kennemer Gasthuis, Haarlem); D van Zeben, MD (Sint Franciscus Gasthuis, Rotterdam). We would also like to thank all other rheumatologists and trainee rheumatologists who enrolled patients in this study and the Sectra Company (Sweden) for estimating BMD of the metacarpals by online digital x-ray radiogrammetry.
REFERENCES
Footnotes
Competing interests: Consultancies: FCB (Schering-Plough Centocor, Merck & Co. Inc., Pfizer Inc., Wyeth, Abbott, Amgen). Honoraria: CFA (Schering-Plough). Grants received: CFA (BeSt study).
Funding: This study was supported by a government grant from the Dutch College of Health Insurances (College Voor Zorgverzekeringen). Schering-Plough BV and Centocor Inc. provided additional grants and supplied the study medication for patients in group 4.