Article Text
Abstract
Objective To evaluate the efficacy of denosumab in suppressing joint destruction when added to conventional synthetic disease-modifying antirheumatic drug (csDMARD) therapy in patients with rheumatoid arthritis (RA).
Methods This was a multi-centre, randomised, double-blind, parallel-group, placebo-controlled phase 3 study in Japan. Patients with RA aged ≥20 years receiving csDMARDs were randomly assigned (1:1:1) to denosumab 60 mg every 3 months (Q3M), denosumab 60 mg every 6 months (Q6M) or placebo. The change in the modified total Sharp score (mTSS) and effect on bone mineral density (BMD) at 12 months was evaluated.
Results In total, 654 patients received the trial drugs. Denosumab groups showed significantly less progression of joint destruction. The mean changes in the mTSS at 12 months were 1.49 (95% CI 0.99 to 1.99) in the placebo group, 0.99 (95% CI 0.49 to 1.49) in the Q6M group (p=0.0235) and 0.72 (95% CI 0.41 to 1.03) in the Q3M group (p=0.0055). The mean changes in bone erosion score were 0.98 (95% CI 0.65 to 1.31) in the placebo group, 0.51 (95% CI 0.22 to 0.80) in the Q6M group (p=0.0104) and 0.22 (95% CI 0.09 to 0.34) in the Q3M group (p=0.0001). No significant between-group difference was observed in the joint space narrowing score. The per cent change in lumbar spine (L1–L4) BMD in the placebo, Q6M and Q3M groups were −1.03%, 3.99% (p<0.0001) and 4.88% (p<0.0001). No major differences were observed among safety profiles.
Conclusions Denosumab inhibits the progression of joint destruction, increases BMD and is well tolerated in patients with RA taking csDMARD.
- rheumatoid arthritis
- denosumab
- joint destruction
- erosion
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Denosumab, an antibody targeting receptor activator of nuclear factor κB ligand, can successfully inhibit the progression of bone erosion and increase bone mineral density (BMD) in patients with rheumatoid arthritis (RA) receiving methotrexate.
What does this study add?
The DESIRABLE study is the largest study performed to date investigating the efficacy of denosumab in patients with RA.
Denosumab significantly inhibits the progression of joint destruction: denosumab inhibited the progression of the modified total Sharp score and erosion scores and led to an increase in BMD but did not affect the joint space narrowing or disease activity scores in patients with RA receiving conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) (including methotrexate).
Denosumab was generally well tolerated in patients receiving background treatment with csDMARDs.
How might this impact on clinical practice or future developments?
Denosumab has potential as a novel therapeutic option for suppression of bone erosion and bone loss in patients with RA with or without concomitant osteoporosis, particularly in patients who are contraindicated for biological disease-modifying antirheumatic drugs (bDMARDS).
Introduction
Rheumatoid arthritis (RA) is characterised by inflammatory synovitis that causes joint cartilage and bone destruction1 2 and increases fracture risk through bone erosion and osteoporosis.3 4 Bone damage is localised to the periarticular cortical areas of inflamed joints in early RA, but osteoporosis extends to the diaphyses becoming generalised in advanced stages. On infiltration into the periarticular region, activated T and B cells express two essential osteoclast mediators, one being the receptor activator of nuclear factor κB ligand (RANKL).1 2 5 The proliferated synovium erodes from the osteochondral junction into the bone tissue, with osteoclasts destroying the local joint site.
Disease-modifying antirheumatic drugs (DMARDs) modulate the inflammatory immune responses slowing radiographic damage.6 Conventional synthetic DMARDs (csDMARDs) are commonly prescribed for patients with RA, although methotrexate remains the gold standard. Biological DMARDs (bDMARDs) are also used to target specific proteins and can potently suppress RA disease; however, they may cause serious infection. Some patients do not respond fully to bDMARDs,1 possibly because their joint destruction is unconnected to the clinical scores of inflammation.7 These issues may be compounded by the worsening of RA pathology by steroid-induced and disuse osteoporosis.8
RANKL is essential for osteoclast development, activation and survival.9 Denosumab, a fully human monoclonal IgG2 antibody, binds and neutralises the activity of human RANKL suppressing bone resorption10 and may also prevent progression of bone erosion. Two phase 2 studies of denosumab have been conducted in patients with RA receiving methotrexate: in a study in the USA and Canada, denosumab 60 or 180 mg was administered every 6 months,11 while in a Japanese study, denosumab 60 mg was administered every 2, 3 or 6 months.12 Both demonstrated significant inhibition of bone erosion progression compared with placebo. Therefore, we conducted this phase 3 study to evaluate the effect of denosumab on the progression of joint damage in patients with RA being treated with csDMARDs.
Methods
Study design and patients
This was a randomised, double-blind, parallel-group, placebo-controlled phase 3 study conducted at 104 hospitals in Japan. We enrolled patients aged ≥20 years who fulfilled the 1987 American College of Rheumatology (ACR) criteria13 or 2010 ACR/European League Against Rheumatism criteria14 for RA, who had RA for 6 months to <5 years and were receiving treatment with one or more csDMARDs. Based on a treat-to-target strategy, investigators adjusted csDMARD dosages for RA disease activity and/or added other treatments as appropriate (including nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids and csDMARDs such as salazosulfapyridine, bucillamine and tacrolimus in addition to methotrexate). Eligible patients had bone erosions (≥1) on radiographs or met the following criteria: C-reactive protein (CRP) levels of ≥1.0 mg/dL or an erythrocyte sedimentation rate (ESR) of ≥28 mm/hour, and positive anticyclic citrullinated peptide antibodies or rheumatoid factor (>20 IU/mL). The main exclusion criteria were RA functional class IV15 and previous or current treatment of RA with any biological agents. Use of bisphosphonates, parathyroid hormones, oral glucocorticoids (prednisolone equivalent doses of >10 mg/day), biologics and tofacitinib6 were prohibited. However, hormone replacement therapy was allowed. Participants continued csDMARDs treatment and received ≥600 mg/day calcium with vitamin D.
Treatment and randomisation
The study drugs were denosumab (one prefilled syringe containing 1 mL of a sterile colourless solution of denosumab 60 mg) and denosumab-matching placebo. Patients received either denosumab or placebo at 0, 3, 6 and 9 months.
Patients were stratified by baseline glucocorticoid use and randomly assigned in a 1:1:1 ratio by the Interactive Web Response System to receive one of three treatments for 12 months: subcutaneous injection of denosumab 60 mg every 6 months (Q6M), every 3 months (Q3M) or placebo. Assignment was done using the RANUNI function of SAS software (release V.9.1.3). Treatment was masked to patients, investigators, sponsors and trial personnel involved in measuring outcomes.
Collected patient data and assessments
Radiographs were taken at baseline, 6 months and 12 months and reviewed at a central imaging core lab. Independent readers evaluated hand and foot radiographs by the modified Sharp/van der Heijde method.16 Dual energy X-ray absorptiometry scans for bone mineral density (BMD) were performed to evaluate the lumbar spine (L1–L4) at baseline and 12 months, using Hologic Inc (USA) or GE Healthcare Ltd (UK) devices, and analyses were performed by BioClinica Inc (USA).
Clinical assessments at baseline, 6 months and 12 months included: physician’s and patient’s global assessment of disease activity by visual analogue scale (VAS), patient’s assessment of pain by VAS, Health Assessment Questionnaire-Disability Index (HAQ-DI),17 the 66-joint count for swollen joints and the 68-joint count for tender joints. Blood and urine samples were obtained at fixed times and analysed at a central laboratory (LSI Medience Corporation, Tokyo, Japan) for haematology, blood biochemistry, serum C-telopeptide of type I collagen (CTX-I), urine C-telopeptide of type II collagen (CTX-II) and serum cartilage oligomeric matrix protein (COMP). CTX-II was adjusted for creatinine (CTX-II/Cre). The ACR response18 and disease activity score 28 (DAS28) CRP and ESR19 were calculated using these outcomes. Antidenosumab antibodies were assessed at baseline and 12 months by PPD Development, LLC (USA).
Outcome measures
The primary endpoint was the change in the modified total Sharp score (mTSS) from baseline to 12 months. Secondary endpoints were the changes from baseline to 6 months in the mTSS, changes in the erosion score at 6 months and 12 months, changes in the joint space narrowing (JSN) score at 12 months, per cent change in lumbar spine (L1–L4) BMD and the per cent change in markers of bone and cartilage metabolism. Exploratory variables of efficacy were assessed at 12 months: the proportion of patients without radiographic progression (change in the radiographic score of ≤0.5)20 according to the mTSS, the erosion score or the JSN score; the per cent change in BMD in the subgroup using glucocorticoids or the subgroup with baseline osteoporosis; and the proportions of patients achieving an ACR20/50/70 response, a change in HAQ-DI, DAS28-CRP and DAS28-ESR from baseline. Safety was assessed by adverse events, laboratory tests and antidenosumab antibody levels and summarised according to the Medical Dictionary for Regulatory Activities (V.19.0).
Statistical analyses
A sample size of 214 patients per group was estimated to be sufficient to obtain a power of 0.9 and a two-sided significance level of 0.05, assuming a probability of 0.593 that an individual change in mTSS score randomly drawn from the denosumab group is less than that from the placebo group (based on the phase 2 study12) and allowing 5% missing radiographic data. The full analysis set (FAS) for the efficacy analyses included all patients who received the assigned study drug and had an available mTSS score at baseline and at least one postbaseline assessment. Missing values for the radiographic score were imputed by linear extrapolation/interpolation. For other variables, no imputation was implemented.
The primary analysis compared the change in mTSS from baseline to 12 months between each denosumab group and the placebo group using the van Elteren stratified rank test adjusted for randomised strata (baseline glucocorticoid use). A hierarchical testing procedure was used to control the overall type I error at 0.05 for multiple comparisons of the primary endpoint; if there was a significant difference between the Q3M and placebo groups, comparison between the Q6M and placebo groups was formally tested. The same analysis method was used without multiple adjustments for the other radiographic score endpoints.
The proportions of patients without radiographic progression, with rapid radiographic progression (yearly progression of ≥5 in mTSS), and of patients achieving ACR20/50/70 were analysed using the Cochran-Mantel-Haenszel test adjusted for randomised strata. In a post hoc analysis, the proportion of patients with minimal clinically important progression of HAQ-DI (change from baseline ≥0.22)21 was analysed by the same method. For changes from baseline in the DAS28-CRP, DAS28-ESR and HAQ-DI, comparisons of each denosumab group with the placebo group were performed using repeated measures analysis adjusted for treatment, visit, baseline value, randomised strata and treatment-by-visit interaction. The per cent changes from baseline in lumbar spine (L1–L4) BMD, including for subgroups, were assessed using the analysis of covariance model. Safety was analysed with the data set from patients who received at least one dose of the study drug. All analyses were conducted using SAS V.9.2.
Results
Patient disposition and baseline characteristics
Between 29 October 2013 and 1 December 2015, 679 patients were randomised (figure 1). The FAS included 654 patients. One patient in the Q6M group received placebo by mistake, and therefore, that patient’s safety data were included in the placebo group. Baseline characteristics were similar across treatment groups (table 1). Dose adjustments of csDMARD and glucocorticoid were made similarly among treatment groups.
Baseline demographics and characteristics
Trial profile. *As one patient assigned to denosumab Q6M was administered placebo by mistake, the patient was included in the placebo group for safety analysis. †No mTSS measurements available at baseline or after the first administration of treatment. §207 (per-protocol set). ¶202 (per-protocol set). ǂ200 (per-protocol set). mTSS, modified total Sharp score; PRT, protocol; Q3M, every 3 months; Q6M, every 6 months.
Efficacy
Regarding the primary endpoint, the mTSS increased in all groups despite csDMARD treatment (figure 2A). The Q3M and Q6M groups showed significantly smaller changes in the mTSS from baseline to 12 months versus the placebo group: mean changes were 1.49 (95% CI 0.99 to 1.99) in the placebo group; 0.99 (95% CI 0.49 to 1.49), Q6M group (p=0.0235); and 0.72 (95% CI 0.41 to 1.03), Q3M group (p=0.0055). Compared with placebo, changes in the mTSS from baseline to 6 months were significantly smaller in the Q6M and Q3M groups (p=0.0360 and 0.0028, respectively) (figure 2A), as were the changes in the erosion score from baseline to 12 months (p=0.0104 and 0.0001, respectively). At 6 months, only the Q3M group showed a significantly lower result in the erosion score versus placebo (p=0.0002) (figure 2B). Overall, the changes in the JSN score from baseline to 12 months were not significantly different among groups (figure 2C).
Mean changes from the baseline in the radiographic scores by the van der Heijde-modified Sharp method. (A) Modified total Sharp score, (B) modified Sharp erosion score and (C) modified Sharp joint space narrowing score. Missing values were imputed using linear extrapolation/interpolation. Mean and 95% CIs are presented. P values were calculated by two-sided van Elteren stratified rank test adjusting for baseline use of glucocorticoid. BL, baseline; n, number of patients who received ≥1 dose of investigational product and had a baseline and at least one postbaseline measurement of the radiograph score; Q3M, every 3 months; Q6M, every 6 months.
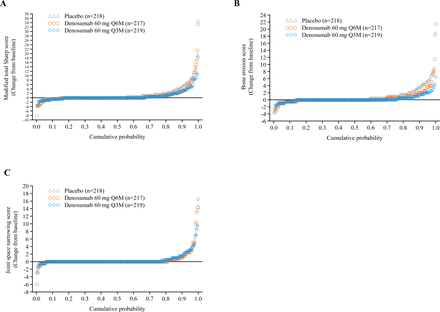
Cumulative probability plots for changes in the mTSS, erosion score and JSN score at 12 months are shown in figure 3. The proportions of patients without radiographic progression at 12 months were greater in the denosumab groups versus the placebo group: 64.2% in the placebo group (140/218), 75.6% in the Q6M group (164/217; p=0.0097) and 78.1% in the Q3M group (171/219; p=0.0014). Likewise, significant increases in the proportions of patients without progression of radiographic erosion (change in erosion score ≤0.5) were observed in both denosumab groups. The proportions of patients with progression of JSN were similar among the groups. The proportions of patients with rapid radiographic progression at 12 months in the placebo, Q6M and Q3M groups were 10.6% (23/218), 6.9% (15/217; p=0.1785 vs placebo) and 5.0% (11/219; p=0.0310 vs placebo), respectively.
Cumulative probability plots of changes from the baseline in the radiographic score at 12 months. (A) Modified total Sharp score, (B) modified Sharp erosion score and (C) modified Sharp joint space narrowing score. n, number of patients who received ≥1 dose of investigational product and had a baseline and at least one postbaseline measurement of the radiograph score; Q3M, every 3 months; Q6M, every 6 months.
The per cent changes in lumbar spine (L1–L4) BMD from baseline to 12 months were −1.03%, 3.99% and 4.88% in the placebo, Q6M and Q3M groups, respectively (p<0.0001 for both denosumab groups vs placebo) (figure 4A). Lumbar spine (L1–L4) BMD was significantly increased in the denosumab groups versus the placebo group, regardless of glucocorticoid use or osteoporosis (p<0.0001 for both subgroups vs placebo) (figure 4B and C).
Per cent change in lumbar spinal bone mineral density (BMD) at 12 months from baseline in all patients (A), patients stratified by baseline use of glucocorticoid (B) and patients stratified by osteoporosis status (C). Data are for full analysis set (observed data). Coloured bars show least square mean values. P values are calculated using the analysis of covariance model after adjusting for treatment, baseline value, machine type, baseline value-by-machine type interaction and baseline use of glucocorticoid. Q3M, every 3 months; Q6M, every 6 months.
Denosumab treatment significantly decreased the bone metabolism marker CTX-I level at 1 month, and the decrease was maintained over the study period (online supplementary file 1A). Urine CTX-II/Cre was decreased by both doses of denosumab for the first 3 months; thereafter, a decrease was observed only in the Q3M group (online supplementary file 1B). However, denosumab showed no effect on the cartilage turnover serum marker COMP (online supplementary file 1C).
Supplemental material
No significant differences were observed between groups in the ACR20/50/70 at 12 months (online supplementary file 2). No significant changes in the DAS28-CRP or DAS28-ESR at 12 months were observed. Changes in the HAQ-DI at 12 months were only significant in the Q6M group (−0.09, p=0.0028). Post hoc analysis revealed that the proportions of patients with minimal clinically important progression of HAQ-DI (≥0.22)21 were lower in both denosumab groups versus the placebo group.
Supplemental material
Safety
The incidence of all adverse events was similar among the groups, except for stomatitis, which was observed more frequently in the denosumab groups (table 2). One death was reported due to interstitial lung disease in the Q3M group. This was judged to be drug related; however, the patient was known to have rheumatic interstitial pneumonia concomitantly.
Summary of adverse events
Discussion
RA is a systemic autoimmune inflammatory disease that causes joint destruction and osteoporosis, primarily through osteoclast activation.3 4 We conducted this phase 3 study to evaluate the efficacy and safety of denosumab in patients with RA, in whom disease activity can be controlled by 1 year treatment with csDMARDs irrespective of whether they were receiving glucocorticoids. In this study, denosumab significantly inhibited the progression of joint destruction and increased lumbar spine BMD in patients receiving concomitant csDMARD treatment. These results are consistent with those of previous research in patients with RA.11 12
Overall efficacy assessment
Although the effects of Q3M versus Q6M regimens were not compared in this study, the data for the Q3M regimen were better than those for the Q6M regimen in all the mean changes at 12 months, the percentage of progression-free patients (ΔmTSS≤0.5), the proportion of rapid radiographic progression and the per cent change in BMD. Notably, progression of mTSS was lower with the Q3M regimen versus the Q6M regimen, suggesting that the Q3M regimen is numerically more effective.
Progression of joint damage is irreversible, and the small amounts accrued over 1 year can gradually accumulate, causing extensive damage. Moreover, radiographic damage is related to clinical outcomes important to patients such as work and physical function. Therefore, preventing joint destruction is important. Denosumab suppressed the mean change in mTSS (0.72 in the Q3M group) and increased the proportion of patients without mTSS progression (78.1% in the Q3M group). However, true clinical relevance cannot be assessed over the short duration of a trial.
Comparison with studies of bDMARDS is difficult as demographic factors and populations are different. However, the Certolizumab–Optimal Prevention of joint damage for Early RA study of certolizumab reported a mean change in mTSS in week 52 of 1.58 and 0.36, and a proportion of subjects with mTSS ≤0.5 of 70.7% and 82.9% in the placebo and certolizumab groups, respectively.22
Denosumab suppressed joint margin erosion but did not block JSN or affect the proportion of patients without JSN change, compared with placebo. Furthermore, we found no significant differences in key indicators of disease activity. Denosumab did not affect the cartilage turnover marker serum COMP, although a clear inhibition was observed for bone metabolism markers. These results were consistent with past studies.11 12 Accordingly, distinct from DMARDs, we can conclude that denosumab did not exert an anti-inflammatory effect in patients with RA. These observations were expected, given that denosumab has only been shown to inhibit RANKL–receptor activator of nuclear factor κB (RANK) signalling during osteoclastogenesis, without affecting other inflammatory pathways.10
Functional assessment
The HAQ-DI assesses physical functional status in adults with arthritis and correlates with the disease activity score.23 In this study, no clinically meaningful improvement in the HAQ-DI was obtained, consistent with the finding that denosumab has no effect on disease activity. As joint destruction assessed by the mTSS correlates with functional disability over time,24 denosumab seems likely to have a preferential effect on physical function. Therefore, evaluating the long-term effects of denosumab in this area may be worthwhile.
Effects on lumbar spine BMD
Individuals with RA have an increased risk of bone loss and fracture.3 4 In this study, denosumab prevented a decrease in lumbar spine BMD and significantly increased the BMD. This increase was observed regardless of glucocorticoid use or baseline osteoporosis, as has been reported previously.25 Patients at risk of osteoporosis tend to be treated with bisphosphonates, which inhibit osteoclastic bone resorption,26 but non-adherence reduces its efficacy.27 Annually administered parenteral zoledronate could be superior to oral bisphosphonates due to improved compliance; notably, only zoledronate has been reported to suppress progression of bone erosions in patients with RA.28 One study showed that denosumab prevents bone erosion, but alendronate had no effect.29 Another reported that denosumab was more effective than zoledronate for menopause-related osteoporosis.30 The differences in effects between bisphosphonates and denosumab may be explained by their distributions and mechanisms of action.31 Bisphosphonates are preferentially distributed in the gaps of bone resorption present in trabecular bone, taken up by osteoclasts and inhibit bone resorption with greater effect on cancellous bone. In contrast, denosumab is distributed throughout the extravascular space, without binding to the bone surface, inhibiting bone remodelling in both cortical and cancellous bone by blocking the RANKL–RANK interaction, a common pathway for osteoclast differentiation in osteoporosis and inflammation. Denosumab is the first drug shown to have potent suppressive effects on both osteoporosis and joint destruction in patients with RA who are concomitantly receiving csDMARDs. As osteoporosis and joint destruction have been observed in some patients with RA treated with bDMARDS,32 and because bDMARDs are contraindicated in some patients due to infection risk or costs, a combination of csDMARDs and denosumab could be a new treatment option for patients in whom csDMARDs are used to control disease activity.
Safety profile
The incidence of adverse events was similar across treatment groups. Specifically, hypocalcemia, an identified risk of denosumab,33 and fractures were comparable between groups. Overall, the safety profile of each denosumab group was broadly comparable to the placebo group, consistent with previous studies.11 12 33
Study limitations
This study has some limitations, including the prohibited concomitant use of biologics and tofacitinib and the short study duration. This short duration was chosen for ethical reasons: to treat patients optimally in the placebo group. We conducted an open-label extension study following the phase 3 study to evaluate long-term effects and are planning a follow-up study of patients who completed the phase 3 extension study to assess the rebound effects and safety of denosumab on bone erosion and BMD in patients with RA.
Clinical impact and future potential
Currently, not all patients with RA receive treatment to sufficiently suppress joint destruction for many reasons. Due to safety concerns, csDMARDs have a dose limit, and some csDMARDs cannot be combined. Furthermore, biologics are not used in all patients who insufficiently respond to csDMARDs for safety or economic reasons, even if indicated.34 In clinical trials using denosumab, no additive anti-inflammatory effect was observed in patients with RA, but its inhibitory effect on joint destruction was confirmed by use in combination with csDMARDs. Hence, we believe that this drug is a novel treatment option for patients with RA who do not respond well to csDMARDs, are unable to adjust or start other DMARDs due to safety concerns or costs or require treatment for osteoporosis.
References
Footnotes
TT and YT contributed equally.
Handling editor Josef S Smolen
Contributors TT and YT contributed equally as first authors. TT, YT, SS and HY provided substantial contributions to the study conception and design. TY diagnosed the oral adverse events. ST diagnosed the atypical femoral fracture events. TN was involved in the design and conduct of the study and in collecting data. NO was involved in the design of the study and data analysis. DvdH supervised scoring of the radiographs. All authors interpreted the data. All authors discussed and agreed on the content of the manuscript before submission.
Competing interests TT has received research grants from AbbVie, Asahi Kasei, Astellas, AYUMI, Chugai, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Nippon Kayaku, Novartis, Pfizer and Takeda and has received personal fees from AbbVie, Astellas, Astra Zeneca, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, Nippon Kayaku, Novartis, Pfizer, Sanofi, Taiho, Taisho Toyama, Takeda, Teijin and UCB. YT has received research grants from AbbVie, Astellas, Chugai, Bristol-Myers Squibb, Daiichi Sankyo, Eisai, Kyowa Hakko Kirin, Mitsubishi Tanabe, MSD, Ono, Pfizer and Takeda and has received personal fees from Astellas, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eli Lilly, Janssen, Mitsubishi Tanabe, Pfizer, Sanofi, UCB and YL Biologics. SS has received grant/research support from Chugai and Daiichi Sankyo and has received personal fees from Asahi-Kasei Pharma, Astellas, MSD, Chugai, Daiichi Sankyo, Eli Lilly, Mitsubishi-Tanabe, Pfizer, Takeda and Teijin. HY has received research grants from AbbVie, Astellas, AYUMI, BMS, Chugai, Daiichi Sankyo, Eisai, Kaken, Mitsubishi Tanabe, MSD, Nippon Shinyaku, Ono, Pfizer, Takeda, Teijin, Torii and UCB and has received consulting fees from Astellas, BMS, Chugai, Daiichi Sankyo, Mitsubishi Tanabe, Nippon Kayaku, Pfizer, Takeda, Teijin and YL Biologics. TY has received Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT #17H04377) and has received consulting fees from Daiichi Sankyo. ST has acted as a consultant for AbbVie, Asahi Kasei Pharma, Amgen, Astellas, Daiichi Sankyo, Eli Lilly, MSD, Ono and Teijin Pharma. TN is an employee of Daiichi Sankyo. NO is a shareholder and employee of Daiichi Sankyo. HKG has received consulting fees from Amgen, Agnovos, Bioclinica, Biomarin, Clementia, Daiichi Sanyo, Eli Lilly, Janssen, Medimmune, Merck, Novartis, Pfizer, Regeneron, Servier and Takeda. DvdH has received consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda and UCB and is the director of Imaging Rheumatology BV.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Deidentified individual patient data and supporting documents pertaining to this study, such as the study protocol, statistical analysis plan and clinical study report, are provided upon request via the data sharing portal (https://vivli.org/ourmember/daiichi-sankyo/) in accordance with the data sharing policy of Daiichi Sankyo Co., Ltd.